AUTHOR: Anthony G. Thomas, DO, FACP
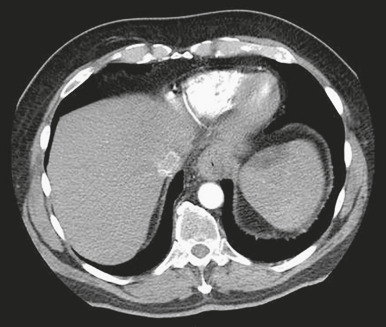
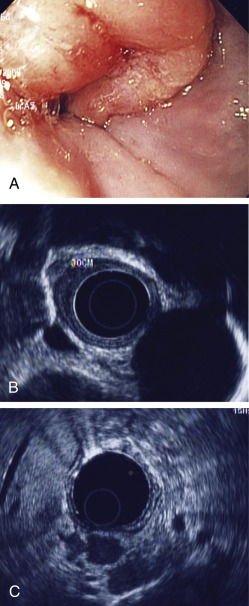
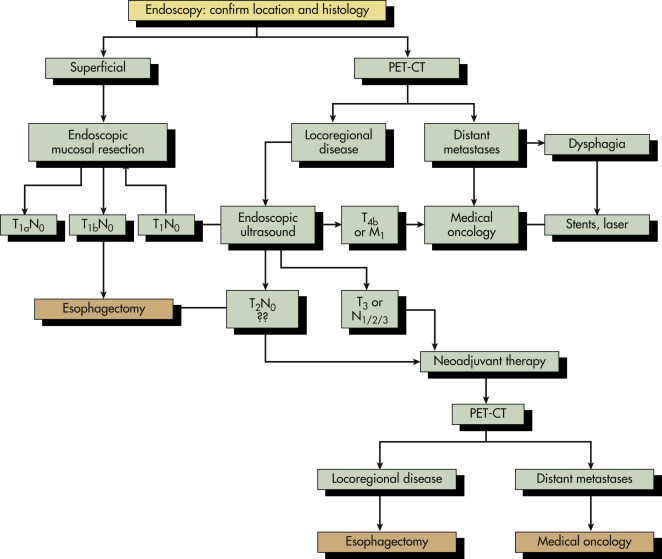
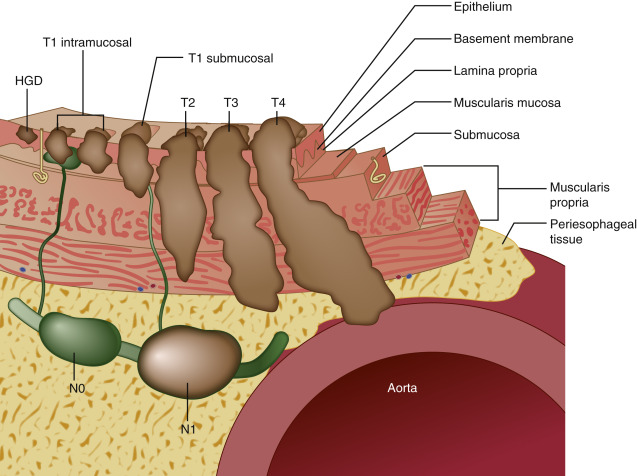
Esophageal tumors include benign and malignant neoplasms of the esophageal mucosa and wall. Carcinomas of the esophageal epithelium, both squamous cell carcinoma and adenocarcinoma (including adenoacanthoma, mucoepidermoid, and adenoid cystic), are the most common tumors of the esophagus. Rare esophageal tumors include both malignant (spindle cell, small cell, sarcoma, lymphoma, melanoma, and choriocarcinoma) and benign neoplasms (leiomyoma, papilloma, and fibrovascular polyps). One can also develop metastatic disease from a cancer that originated in another organ, but this is very rare. Breast cancer, lung cancer, and melanoma would be the most likely culprits. Other cancers can directly extend to the esophagus from the larynx, pharynx, lung, thyroid, or stomach. Approximately 15% of esophageal tumors arise in the proximal esophagus, 50% in the middle third of the esophagus, and 35% in the lower third. Tumors involving the esophageal-gastric junction are usually staged and treated as esophageal cancers if the tumor epicenter is no more than 2 cm into the proximal stomach. Tumors in the upper two thirds are usually squamous cell cancers, and tumors in the lower third are usually adenocarcinomas. The incidence of superficial esophageal cancers is increasing. These invade no deeper than the submucosa.
| ||||||||||||||||||||||||
It is the eighth most common cancer worldwide and the seventh leading cause of cancer deaths. Rates are increasing every decade and are highest in the Asian esophageal cancer belt, extending from the Caspian Sea to northern China, with certain high-incidence pockets in Finland, Ireland, southeast Africa, and northwest France. Incidence has increased six-fold since 1975. Foods including cured meats and spicy regional fare likely play a role in certain areas. Increasing substance abuse with tobacco and alcohol also coincides with a rise in esophageal cancers. Rates of squamous cell carcinoma are decreasing while those of adenocarcinomas are dramatically increasing. The direct causes of squamous cell carcinoma most commonly include tobacco and alcohol abuse. Epithelial dysplasia usually occurs, which progresses to carcinoma in situ. Adenocarcinomas are usually the result of gastroesophageal reflux disease (GERD) and obesity. The mucosa of the esophagus undergoes intestinal metaplasia. Genetic alterations occur and perpetuate during proliferation.
In the U.S., there were an estimated 16,000 new cases and 15,000 deaths are reported yearly, making it the seventh leading cause of death by cancer among men. The majority of cases are diagnosed at an advanced stage (unresectable or metastatic disease).
In the U.S., squamous cell esophageal cancer is more common among African Americans compared to whites, whereas adenocarcinoma more common in whites. The overall male:female ratio is 3 to 4:1; the highest male:female ratio is in the Hispanic population. Usually develops in fifth to seventh decades and associated with lower socioeconomic status.
Increasing evidence shows that genetics may play a role by increasing susceptibility to esophageal cancer. One well-identified disease associated with esophageal cancer is tylosis (focal nonepidermolytic palmoplantar keratoderma), linked to loss of heterozygosity on chromosome 17q. Familiar clustering of Barrett esophagus and the recent identification of germline mutations in affected sibling pairs support a genetic link to esophageal adenocarcinoma. Also, up to 35% of esophageal adenocarcinomas can have overexpression of HER2, which can lead to the use of targeted therapy (trastuzumab) in the treatment plan.
- Dysphagia (74%): Initially with solid foods, gradually progresses to semisolids and liquids; latter signs usually indicate incurable disease with tumor involving more than 60% of the esophageal circumference. It may be felt as chest pain
- Unintentional weight loss of short duration. Loss >10% body mass predicts poor outcome
- Hoarseness: Suggests recurrent laryngeal nerve involvement
- Odynophagia and halitosis: Unusual symptoms
- Cervical adenopathy: Usually involving supraclavicular lymph nodes
- Dry cough: Suggests tracheal involvement
- Aspiration pneumonia: Caused by fistula between the esophagus and trachea
- Iron deficiency anemia: Related to chronic GI blood loss
- Massive hemoptysis or hematemesis from the invasion of vascular structures
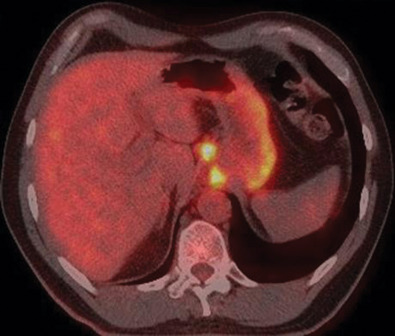
- Advanced disease spreads to lymph nodes, liver, lungs, peritoneum, and pleura
- Hypercalcemia: Associated with squamous cell carcinoma from secretion of a parathyroid-like tumor peptide
The pathogenesis of esophageal cancers is attributable to chronic recurrent oxidative damage from any of the following etiologic agents, which cause inflammation, and esophagitis, increased cell turnover, and, ultimately, initiation of the carcinogenic process.
- Excess alcohol consumption is strongly associated with squamous cell esophageal cancer in the U.S.; hard liquor is associated with a higher incidence than wine or beer
- Tobacco and alcohol synergistically increase risk for squamous cell cancer
- Other ingested carcinogens:
- Mucosal damage
- Radiation-induced strictures
- Achalasia: Incidence of esophageal cancer is seven times greater in this population
- Host susceptibility as a result of precancerous lesions:
- Plummer-Vinson syndrome (Paterson-Kelly): Glossitis with iron deficiency
- Congenital hyperkeratosis and pitting of palms and soles (tylosis)
- Human papillomavirus infection (types 16 and 18) has been detected in squamous cell carcinoma of the esophagus, sometimes associated with p53 tumor suppressor gene mutations
- Possible relationship with prolonged bisphosphonates (≥10 prescriptions, or >3 yr use)
- Possible association with celiac sprue or dietary deficiencies molybdenum, selenium, zinc, vitamin A
- The incidence of adenocarcinoma is continually rising.
- Smoking increases risk of adenocarcinoma, particularly in patients with Barrett.
- Obesity, hiatal hernia, and diets lacking in fresh fruit and vegetables and high in fat (particularly from red meat and processed foods) increase risk.
- Chronic GERD leading to Barrett metaplasia and adenocarcinoma via immune cell infiltration and production of inflammatory mediators and reactive oxygen species. The annual rate of transformation from Barrett to adenocarcinoma is <0.5%.
- Helicobacter pylori infection may reduce risk of adenocarcinoma but can increase risk of lymphoma.