AUTHORS: Harlan G. Rich, MD, FACP, AGAF and Rei Mitsuyama, MD



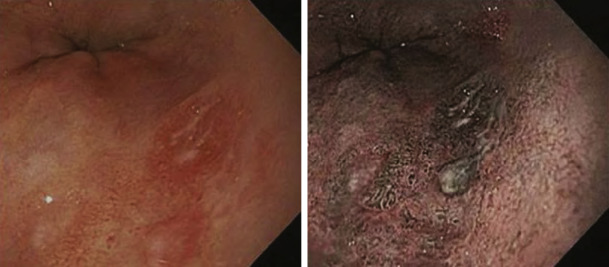
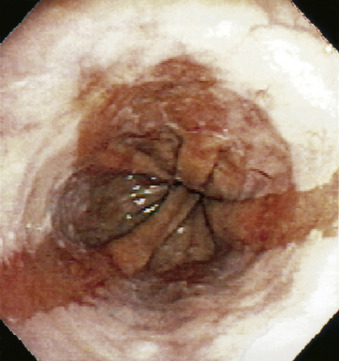
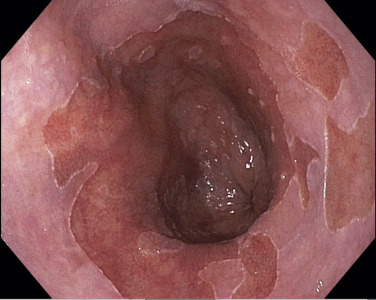
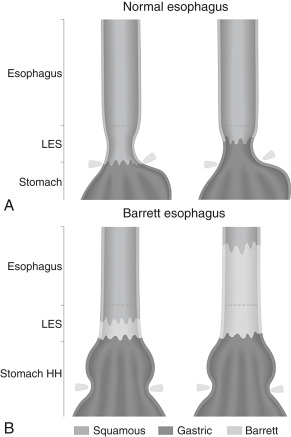
DefinitionBarrett esophagus occurs when the squamocolumnar junction is displaced ≥1 cm proximal to the gastroesophageal junction and the squamous lining of the lower esophagus is replaced by metaplastic columnar epithelium, which predisposes to the development of esophageal adenocarcinoma. Although cardia-type epithelium has been shown to predispose to esophageal cancer, the presence of intestinalized epithelium is still considered essential for the diagnosis. Recent data show that the absolute annual risk for esophageal carcinoma in Barrett esophagus is 0.12%, which is much lower than the assumed risk of 0.5% that is the basis for current surveillance guidelines.1-3
SynonymsEsophagus, Barrett
Esophagus, columnar-lined
Ulcer, Barrett
| ICD-10CM CODES | | K22.70 | Barrett esophagus without dysplasia | | K22.710 | Barrett esophagus with low-grade dysplasia | | K22.711 | Barrett esophagus with high-grade dysplasia | | K22.719 | Barrett esophagus with dysplasia, unspecified |
|
Epidemiology & Demographics
- Male:female ratio of 4:1.
- Mean age of onset is 40 yr, with a mean age range of diagnosis of 55 to 60 yr.
- Occurs more frequently in white and Hispanic individuals than in African American individuals, with a ratio of 10 to 20:1.
- Mean prevalence of 5% to 15% in patients undergoing endoscopy (esophagogastroduodenoscopy [EGD]) for symptoms of gastroesophageal reflux disease (GERD). It is estimated that 5.6% of adults in the U.S. have Barrett esophagus. The prevalence rate may be as low as ∼1% in patients without risk factors and varies based on patient location (Western vs. non-Western countries) and number of risk factors.
- Independent risk factors include chronic reflux (>5 yr), hiatal hernia, age >50 yr, male sex, white ethnicity, smoking history, and intraabdominal obesity. A family history of at least one first-degree relative with Barrett esophagus or adenocarcinoma of the esophagus is associated with a threefold to fivefold increased risk of Barrett esophagus and esophageal adenocarcinoma.4,5
Physical Findings & Clinical PresentationSymptoms
- Chronic heartburn
- Dysphagia with solid food
- Less frequent: Chest pain, hematemesis, melena
- Patients may be asymptomatic
- May be an incidental finding on EGD in patients without reflux symptoms
Physical Findings
- Nonspecific; can be completely normal
- Epigastric tenderness on palpation
Etiology
- Metaplasia is thought to result from re-epithelialization of esophageal tissue injured as a result of chronic GERD. Activation of proliferative and inflammatory pathways likely induces a molecular phenotypic reprogramming of progenitor cells to form intestinal epithelium. Several candidate cells have been identified, including migrating gastric epithelial cells, residual embryonic cells, transitional columnar epithelium, or submucosal glands or ducts.2,3,4,6
- Patients with Barrett esophagus tend to have more severe esophageal motility disturbances (decreased lower esophageal sphincter pressure, ineffective peristalsis) and greater esophageal acid exposure on 24-h pH monitoring. Table 1 lists some physiologic abnormalities that have been reported in Barrett patients and suggests how these abnormalities may contribute to GERD severity.
- Bile salts in the presence of acid may produce oxidative stress, induce reactive oxygen species, and alter transcriptional factor activity, all of which may induce the formation of Barrett epithelium, DNA damage, and dysplastic progression.
- Familial clustering of GERD and Barrett esophagus suggests a genetic predisposition. Early data suggest that patients who develop Barrett are genetically predisposed to a severe inflammatory response to GERD.
- One meta-analysis of genome-wide association studies identified eight risk loci associated with Barrett esophagus and esophageal adenocarcinoma. Candidate susceptibility loci include CRTC1, BARX1, and FOXP1.7,8
- Progression from metaplasia to dysplasia to carcinoma is associated with accumulating changes in gene structure and expression, including the caudal-related homeobox family of transcription factors (CDX1 and CDX2), the embryonic transcription factor SOX2, and the tumor suppressors p16 (CDKN2A) and TP53. Biomarkers and developing technology like TissueCypher, an image analysis platform, may help better predict cancer progression compared to histology alone, though current studies show low sensitivity and specificity.
- Chromosomal deletions, duplications, and translocation are all potential early events in progression of Barrett esophagus to malignancy.9 A recent study suggests c-MYC and HNF4A as transcriptional drivers of metaplasia.10
- A recent Australian study suggests the presence of four pathologic criteria (loss of surface maturation, mucin depletion, nuclear enlargement, and increase of mitosis), together, was associated with greater progression from low-grade dysplasia to high-grade dysplasia or adenocarcinoma.11
- Transcriptionally active high-risk human papillomavirus has been described in cohorts of patients with Barrett esophagus with dysplasia and esophageal adenocarcinoma.
- Patients with Barrett esophagus and esophageal adenocarcinoma may have a shift in the esophageal microbiome toward a gram-negative population, which may play a role in oncogenesis through the release of proinflammatory cytokines and activation of oncogenic transcriptional regulators such as nuclear factor-κB (NF-κB).12,13
TABLE 1 Proposed Physiologic Abnormalities Contributing to Gastroesophageal Reflux Disease in Patients With Barrett Esophagus
| Abnormality | Potential Consequences |
|---|
| Extreme hypotension of the lower esophageal sphincter | Gastroesophageal reflux |
| Ineffective esophageal motility | Defective clearance of refluxed material |
| Gastric acid hypersecretion | Reflux of highly acidic gastric juice |
| Duodenogastric reflux | Esophageal injury caused by reflux of bile acids and pancreatic enzymes |
| Decreased salivary secretion of EGF | Delayed healing of reflux-damaged esophageal mucosa |
| Decreased esophageal pain sensitivity to refluxed caustic material | Failure to initiate therapy |
EGF, Epidermal growth factor.
From Feldman M et al: Sleisenger and Fordtran’s gastrointestinal and liver disease, ed 10, Philadelphia, 2016, Saunders.

The goal is to control GERD symptoms and maintain healed mucosa.
Nonpharmacologic TherapyNonpharmacologic therapy includes lifestyle modifications; elevating head of bed; and avoiding chocolate, tobacco, caffeine, mints, and certain drugs (see “Gastroesophageal Reflux Disease”).
Acute General Rx
- Proton pump inhibitors are the most effective treatment for GERD. Therapy should be titrated to control symptoms and/or to promote healing of endoscopic signs of disease.22
- If patient is asymptomatic and incidentally found to have Barrett esophagus, once-daily proton pump inhibitors should be prescribed, as they may reduce the risk of neoplastic progression. Increasing dosage to twice daily is only recommended if breakthrough symptoms arise.14
Chronic Rx
- Chronic acid suppression is recommended to control symptoms, maintain healing, and reduce neoplastic progression. For patients with Barrett esophagus, the benefits of the use of proton-pump inhibitors are thought to outweigh the potential risks.14,21,23,24
- Antireflux surgery may be considered for management of GERD and associated sequelae, but it has not been proven to be superior to medical therapy and has no clear role in the management of Barrett esophagus. Barrett patients continue to require endoscopic surveillance of their esophagus.
- When GERD is controlled by either medical or surgical therapy, ablation of metaplastic epithelium usually leads to replacement by normal squamous epithelium. Because only a minority of patients with Barrett esophagus progress to high-grade dysplasia or carcinoma, endoscopic eradication therapy (EET) is not recommended for the general population of patients with nondysplastic Barrett esophagus.16,24,25
- EET is becoming the treatment of choice for low-grade dysplasia and is the treatment of choice for high-grade dysplasia and intramucosal cancer.2,11,25-27 Radiofrequency ablation or photodynamic therapy, combined with endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) of visible mucosal irregularities, should be performed in conjunction with aggressive surveillance and eradication of all remaining Barrett epithelium. Endoscopic therapy is preferred over surgical treatment in properly staged individuals, given lower morbidity and mortality rates and similar long-term survival rates. These therapies may even be considered for patients with focal intramucosal carcinoma, if properly staged (T1 SM1 or lower). Radiofrequency ablation is currently first-line, while cryotherapy (carbon dioxide, liquid nitrogen, or nitric oxide) and argon plasma coagulation are being evaluated for the complete eradication of both dysplasia and intestinal metaplasia and reduced risk for disease progression. All these options run the risk for residual or buried metaplasia. Ultimate goals of therapy include both complete eradication of dysplasia (CE-D) and complete eradication of intestinal metaplasia (CE-IM). Even with CE-IM, recurrence has been documented in 5% to 39.5% of patients. Modalities for endoscopic treatment of Barrett esophagus are summarized in Table 2. Given the high rates of recurrence of Barrett esophagus, those who have undergone successful EET should then continue with endoscopic surveillance.2
- Surgical resection is definitive therapy and may be offered for multifocal high-grade dysplasia, carcinoma that has extended into the submucosa (T1 SM2 or 3), or patients with poorly differentiated carcinomas or with evidence of lymphovascular invasion. Surgery may also be considered in patients with resistant, progressive, or recurrent disease in the face of endoscopic therapy. Mortality appears to be lower with experienced surgeons operating in high-volume centers.
- Patients with cardiovascular risk factors may be considered for low-dose aspirin therapy for chemoprevention of esophageal adenocarcinoma. NSAIDs or NSAIDs combined with statins are potentially effective at reducing the risk of esophageal adenocarcinoma but are not currently recommended for use because of the risk of adverse effects.
TABLE 2 Modalities for Endoscopic Treatment of Barrett Esophagus
| Modality | HGD Resolved | BE Resolved | Recurrence Rate | Subsquamous BE Rate | Stricture Rate | Complication Rate | Advantages | Disadvantages |
|---|
| APC | 67%-98.6% | 38%-98% | 33%-68% low power | 25%-45% low power
0-30% high power | 4%-10% | 24% low power
40%-60% high power | Noncontact, technically simple | Requires several treatment sessions |
| MPEC | - | 25%-88% | 7% | 7% | 2% | 41%-43% | Noncontact, technically simple, relatively inexpensive, readily available | Requires several treatment sessions |
| PDT | 77%-88% | 13% | 5%-11% | 2%-24% | 4.8%-53% | 4.8%-53% | Easy to perform; only FDA-approved ablation method for treatment of precancerous lesions in BE | Photosensitivity, relatively high stricture rate |
| EMR | 59%-97% | 53% | 4%-30% | - | 3%-30% | 12%-60% | Histologic assessment; complete removal of circumferential short-segment BE; 1-2 sessions | Difficulty treating long-segment BE |
APC, Argon plasma coagulation; BE, Barrett esophagus; EMR, endoscopic mucosal resection; FDA, U.S. Food and Drug Administration; HGD, high-grade dysplasia; MPEC, multipolar electrocoagulation; PDT, photodynamic therapy.
From Cameron JL, Cameron AM: Current surgical therapy, ed 12, Philadelphia, 2017, Elsevier.
Disposition
- The relative risk of developing esophageal adenocarcinoma for a patient with Barrett esophagus, as compared with the general population, is 11.3, a substantial drop from the relative risk of 30 or 40 estimated in earlier reports. The risk is greater in men and in patients with longer (≥3 cm) columnar-lined segments.
- The risk of progression to esophageal adenocarcinoma from untreated Barrett with low-grade dysplasia is ∼0.5% per yr, and with high-grade dysplasia ranges from 6% to 19% per yr.
- In the United States and Europe, the Prague criteria are recommended as a means of risk stratification and determination of the frequency surveillance endoscopy. However, adherence is extremely variable.1,28,29
- Prasad et al. developed a model to predict progression of Barrett esophagus to neoplasia (“Progression in Barrett’s oesophagus score”) based on sex, smoking history, length of Barrett’s involvement, and histology.30
- Frequency of monitoring is controversial. No prospective studies have proved that endoscopic surveillance is cost effective or increases life expectancy. Similarly, there are no clear guidelines for when surveillance should be stopped, although the ACG suggests stopping with less than an expected 5-yr survival or when a patient is no longer fit enough to undergo endoscopy or EET.2
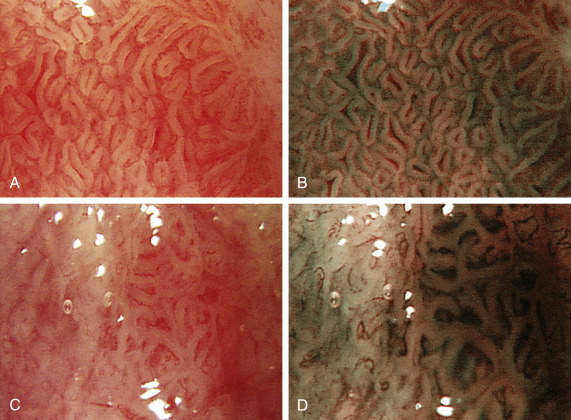
- Patients with Barrett esophagus currently undergo EGD and systematic four-quadrant biopsy (“Seattle protocol”) using white light and chromoendoscopy at intervals determined by the presence and grade of dysplasia. Wide-area transepithelial sampling with computer-assisted 3D analysis (WATS-3D) may increase the detection of dysplasia. All visible mucosal abnormalities should undergo biopsy.14,28
- Patients without dysplasia should have follow-up every 3 to 5 yr (≥3 cm or <3 cm Barrett involvement, respectively).2
- Patients indefinite for dysplasia require aggressive, intensified medical therapy and close follow-up and resampling within 6 mo.
- Patients with low-grade dysplasia should have aggressive antisecretory (proton pump inhibitor) therapy, repeat endoscopy within 2 to 6 mo, and either endoscopic ablation therapy or extensive mucosal sampling at 6 and then every 12 mo thereafter. Patients with high-grade dysplasia should have expert confirmation and extensive mucosal sampling. Advanced imaging techniques should be used to localize all neoplastic lesions. This includes virtual chromoendoscopy, narrow-band imaging (NBI), blue laser imaging (BLI), and i-scan. A simple and inexpensive technique is the use of acetic acid spray, which turns Barrett epithelium white, before turning neoplastic lesions red after 30 to 40 sec. High-grade dysplasia with visible mucosal irregularities should be removed by EMR or ESD, followed by mucosal ablation. Consider intensive surveillance every 3 mo for at least 1 yr and then annually thereafter. Endoscopic treatment is preferred over intensive surveillance in patients with high-grade dysplasia.31
- The ACG recommends after complete eradication that surveillance endoscopy be performed at 1 yr and 2 yr for low-grade dysplasia, and 3, 6, 12 mo and annually for high-grade dysplasia.2 This is less frequent than previously recommended.
- Patients should be treated aggressively for GERD before surveillance.
Referral
- Consider EGD with biopsy in male or selected female patients with multiple risk factors who have not had previous EGD.
- Refer patients with GERD for evaluation if “red flag” symptoms are present (dysphagia, odynophagia, weight loss, vomiting, early satiety, GI bleeding, iron deficiency).
- Refer patients with biopsy-proved Barrett esophagus for surveillance.
- For those with low-grade or high-grade dysplasia, refer for ablative therapy with EMR or ESD if appropriate, followed by intensive surveillance. Esophageal resection may be considered.