AUTHORS: Hannah Fiske, MDand Harlan G. Rich, MD, FACP, AGAF
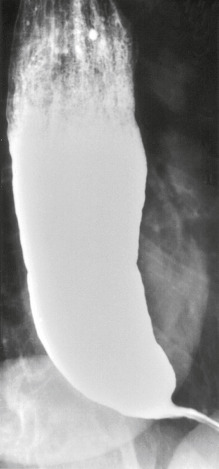
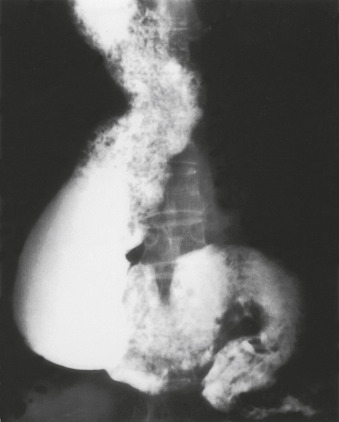
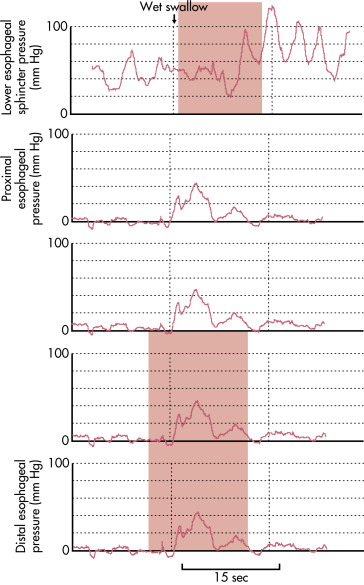
Achalasia is a motility disorder of the esophagus classically characterized by incomplete relaxation of the lower esophageal sphincter (LES) and aperistalsis of the esophageal smooth muscle resulting in functional obstruction of the esophagus.
- Achalasia and cardiospasm
- Achalasia (of cardia)
- Aperistalsis of esophagus
- Megaesophagus
- Esophageal achalasia
- Esophageal cardiospasm
| ||||||||
- Incidence has increased to approximately to 0.03 to 1.63 in 100,000 persons/yr due to studies using high-resolution esophageal manometry (HRM).1
- Prevalence is around 10 per 100,000 persons.
- Although the onset of symptoms may occur at any age, incidence is typically bimodal (between 20 and 40 yr, then after 60 yr) with greater incidence in the elderly.
- Men and women are affected equally.
- Dysphagia (most commonly with both solids and liquids) without oropharyngeal transfer difficulties
- Difficulty belching
- Regurgitation or vomiting of undigested food
- Chest pain and/or heartburn
- Globus
- Frequent hiccups
- Symptoms of aspiration such as nocturnal cough; possible dyspnea and pneumonia
- Weight loss
- The Eckardt symptom score (assessing dysphagia, regurgitation, retrosternal pain, and weight loss) is a fairly reliable measure of achalasia severity and may be used to assess response to therapy
- The Brief Esophageal Dysphagia Questionnaire (BEDQ) is more highly sensitive for manometric diagnosis of dysphagia; it is independent of GERD symptoms and has been shown to be a better symptom-generic evaluating tool, while the Eckardt score is a more achalasia-specific metric.2
- Etiology is poorly understood.
- Loss of intrinsic inhibitory neurons in the myenteric plexus and smooth muscle portion of the esophagus as well as depletion of networks of interstitial cells of Cajal of the LES result in the loss of inhibitory neurotransmitters nitric oxide and vasoactive intestinal polypeptide and unopposed excitatory cholinergic activity, leading to incomplete relaxation of the lower esophageal sphincter (LES) and loss of esophageal peristalsis.1,3
- Loss of myenteric nerve fibers is associated with lymphocytic and eosinophilic infiltrates, capillaritis, plexitis, venulitis, nerve hypertrophy, and fibrosis.
- There is evidence to suggest that esophageal achalasia may be autoimmune mediated, with an increase in immune cells, cytokines, chemokines, and autoimmune antibodies.4This disorder may be caused by autoimmune degeneration of the esophageal myenteric plexus in association with several human leucocyte antigen (HLA) class II DQ antigens. An eight amino-acid insertion in the cytoplasmic tail of HLA-DQβ1 has been identified as a strong achalasia risk factor. Antimyenteric plexus and other antineural autoantibodies have also been described. Patients with achalasia are more likely to have other autoimmune diseases.
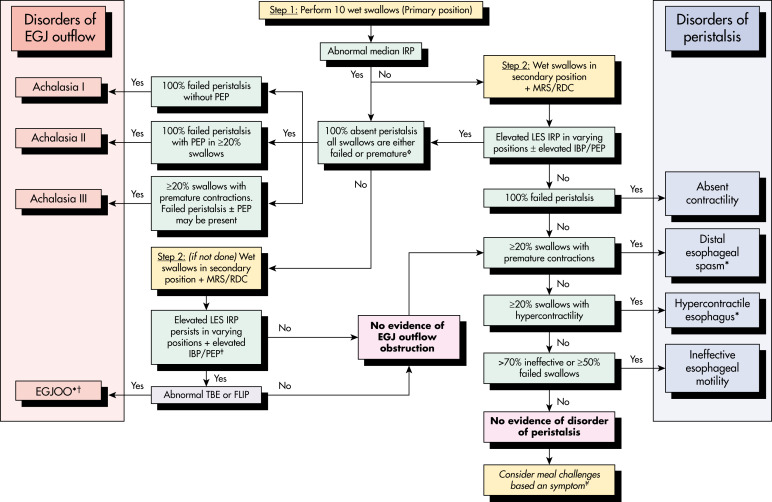
- Abnormal immune reactions to neurotropic viruses, such as varicella zoster, measles, herpes simplex type 1, and human papilloma virus have been implicated. A host T-cell-mediated response may lead to neuronal injury; this has been suggested as the cause of types I and II achalasia (see “Imaging Studies” section).
- Achalasia is also seen in Allgrove syndrome, a rare autosomal recessive disorder caused by gene mutation on chromosome 12q13 and defined by achalasia, alacrima, autonomic disturbance, and acetylcholine insensitivity. Neurons in this syndrome may be susceptible to oxidative injury.
- Recent studies suggest that type III achalasia (see “Imaging Studies” section) is associated with myenteric inflammation but not neuronal loss; downregulation of nitric oxide synthase expression and increased cholinergic sensitivity are cytokine-mediated.
- A type III achalasia pattern has been described in chronic daily opiate users.