Author: Junior Uduman, MD, MS
Acute kidney injury (AKI) is defined as a rapid impairment in kidney function that results in oliguria and retention of nitrogenous products in the blood normally excreted by the kidneys, that is, azotemia. The decline in kidney function can result in volume overload and dysregulation of acid-base status and electrolytes. Current consensus criteria for diagnosis of AKI requires an increase in serum creatinine of 0.3 mg/dl within 48 h or 1.5 times baseline serum creatinine over 7 days, and/or a decline in urine in output to <0.5 ml/kg/h for 6 to 12 h. AKI is further graded by severity as described in Table 1.1
TABLE 1 Consensus Acute Kidney Injury Definitions and Classification Systems
| Serum Creatinine | Urine Output | |
|---|---|---|
| RIFLE Criteria | ||
| Risk | Increase in SCr to ≥1.5 times baseline or decrease in GFR by >25% within 7 days | <0.5 ml/kg/h for >6 h |
| Injury | Increase in SCr to >2 times baseline or decrease in GFR by >50% within 7 days | <0.5 ml/kg/h for >12 h |
| Failure | Increase in SCr to >3 times baseline or decrease in GFR by >75% within 7 days or increase in SCr to ≥4 mg/dl with an acute rise of 0.5 mg/dl | <0.3 ml/kg/h for >24 h or anuria for 12 h |
| Loss | Complete loss of kidney function requiring dialysis for >4 wk | |
| ESRD | Complete loss of kidney function requiring dialysis for >3 mo | |
| AKIN Criteria | ||
| Stage 1 | Increase in SCr by ≥0.3 mg/dl or increase in SCr to ≥1.5 times baseline within 48 h | <0.5 ml/kg/h for ≥6 h |
| Stage 2 | Increase in SCr to >2 times baseline within 48 h | <0.5 ml/kg/h for ≥12 h |
| Stage 3 | Increase in SCr to >3 times baseline within 48 h or increase in SCr to ≥4 mg/dl with a rise of 0.5 mg/dl within 24 h or initiation of dialysis | <0.3 ml/kg/h for ≥24 h or anuria for ≥12 h |
| KDIGO Criteria | ||
| Stage 1 | Increase in SCr by ≥0.3 mg/dl within 48 h or increase in SCr to ≥1.5 times baseline within 7 days | <0.5 ml/kg/h for ≥6 h |
| Stage 2 | Increase in SCr to >2 times baseline within 7 days | <0.5 ml/kg/h for ≥12 h |
| Stage 3 | Increase in SCr to >3 times baseline within 7 days or increase in SCr to ≥4 mg/dl or initiation of dialysis | <0.3 ml/kg/h for ≥24 h or anuria for ≥12 h |
AKIN, Acute kidney injury network; ESRD, end-stage renal disease; GFR, glomerular filtration rate; KDIGO, kidney disease improving global outcomes; RIFLE, risk, injury, failure, loss, end-stage kidney disease; SCr, serum creatinine.
From Newman M et al: Perioperative medicine, ed 2, Philadelphia, 2022, Elsevier.
| ||||||||||||||||||||||||||||||||
- An estimated 20% of hospitalized patients and 60% of intensive care unit patients develop AKI.
- AKI occurs in 20% of patients with moderate sepsis and in >50% of patients with septic shock and positive blood cultures.
- More than 40% of hospital-associated AKI is iatrogenic.
- AKI in hospitalized patients is associated with increased hospital length-of-stay and cost.
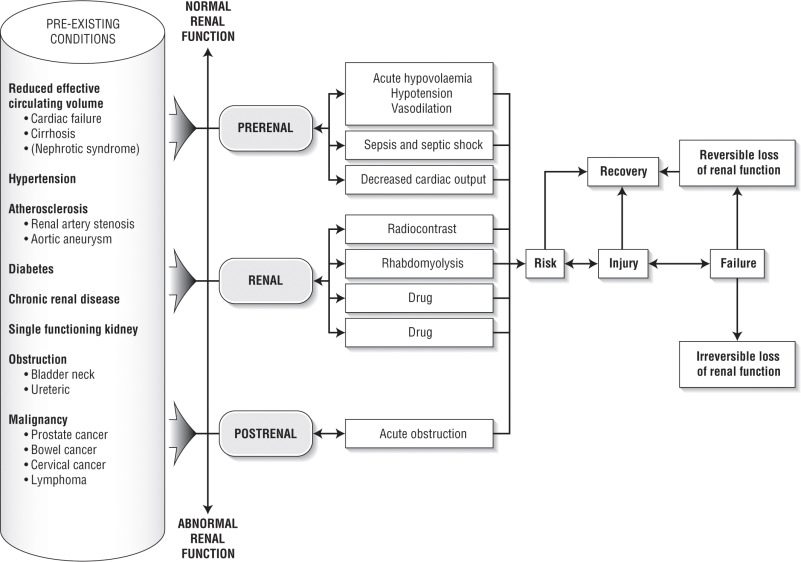
- Most common cause of AKI in hospitalized patients is from intrinsic kidney failure due to acute tubular necrosis (ATN).
- Key risk factors for AKI include older age, preexisting chronic kidney disease, diabetes mellitus, and/or preexisting proteinuria.
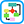
- The clinical presentation of AKI depends on the presence of any preexisting conditions, the underlying conditions, the precipitating event(s) that caused the AKI and the severity of AKI (Fig E1).
- Early or mild AKI is frequently asymptomatic.
- Frequent presenting symptoms include weakness, anorexia, generalized malaise, and nausea.
- Patients may develop oliguric or nonoliguric kidney injury.
- Oliguria is defined as <400 to 500 ml of urine per 24 h. Anuria is frequently seen in ATN or bilateral obstructive uropathy.
- Physical examination should focus on evaluation of volume status, eliciting systemic signs of AKI, and findings supportive of kidney injury etiology.
- Clinical signs and symptoms are numerous; some key findings are highlighted:
- Peripheral edema from volume overload, heart failure, liver failure, or nephrotic syndrome
- Pulmonary edema
- Cardiac dysrhythmias
- Neurologic findings include altered mental status, delirium, lethargy, myoclonus, seizures, and asterixis
- Pruritus, uremic odor
- Flank pain
- Painless hematuria may be seen with glomerulonephritis (GN), whereas painful hematuria is more consistent with obstructive uropathy
- Pericardial effusion and/or pericardial rub
- Fever, skin rash, and arthralgia can be seen with systemic vasculitis
- Classic triad of fever, rash, and eosinophilia in the setting of AKI strongly implicates allergic interstitial nephritis (AIN). However, the simultaneous appearance of all three manifestations occurs in only 30% of cases. When AIN is considered the cause of AKI, a careful review of medications is required
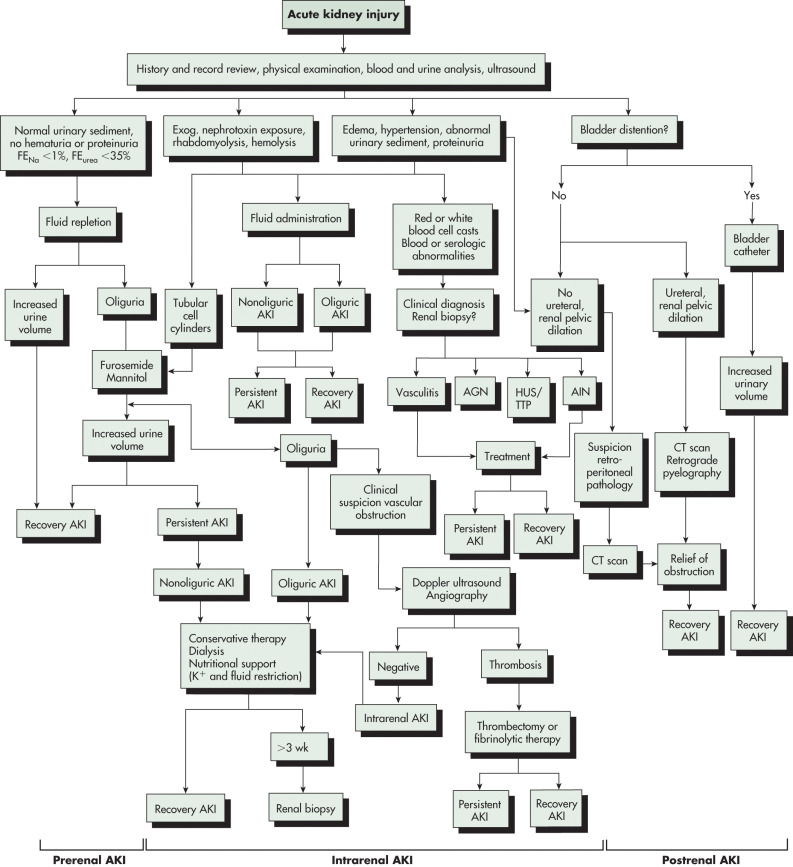
- Prerenal: Inadequate renal perfusion caused by hypovolemia, congestive heart failure (impaired cardiac output), cirrhosis (fluid third-spacing), sepsis (vasodilation), abdominal compartment syndrome, or other. Sixty percent of community-acquired cases of AKI are due to prerenal conditions.
- Postrenal: Bladder outlet obstruction (prostatic enlargement, urethral fibrosis), ureteral obstruction (stones, bladder masses, retroperitoneal fibrosis, ureteral fibrosis), or renal vein occlusion. With two functioning kidneys, bilateral obstruction is usually required to produce significant AKI. Postrenal causes of AKI account for 5% to 15% of community-acquired AKI.
- Intrinsic renal: ATN, AIN, and GN. Common causes of ATN include ischemia (e.g., hypotension or shock, postcardiac bypass, or aorta surgery), rhabdomyolysis, sepsis, drug toxicity (e.g., aminoglycosides, amphotericin, cisplatin), and iodinated radiocontrast-induced nephropathy. Contrast-induced nephropathy is the third most common cause of new-onset AKI in hospitalized patients. However, most of these cases have multiple confounding factors and are better characterized as contrast-associated nephropathy. AIN can develop after exposure to medications, most commonly NSAIDs, antibiotics, and proton pump inhibitors. Microvascular diseases that cause AKI include thrombotic microangiopathies (e.g., thrombotic thrombocytopenic purpura, classic and atypical hemolytic-uremic syndrome, and preeclampsia) and cholesterol emboli.
- Causes of AKI are listed in Table 2.
- Nearly one third of AKI cases may be prevented or mitigated.
TABLE 2 Etiologies of Acute Kidney Injury
| Prerenal Causes (Decreased Renal Blood Flow) | Intrinsic Renal Causes | Postrenal Causes |
|---|---|---|
|
|
|
NSAIDs, Nonsteroidal antiinflammatory drugs; PEEP, positive end-expiratory pressure.
Modified from Cameron JL, Cameron AM: Current surgical therapy, ed 10, Philadelphia, 2011, Saunders.