Return to drug monograph
Drug Name: Zocor 10 MG Oral Tablet
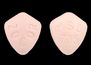
Pill Image:
Ingredient(s): Simvastatin
Imprint: MSD;735
Color(s): Orange
Shape: Oval
Size (mm): 9.00
Score: 1
Inactive Ingredient(s): ascorbic acid / butylated hydroxyanisole / citric acid monohydrate / hydroxypropyl cellulose / hypromellose 2910 (6 cps) / ferric oxide yellow / ferric oxide red / lactose / magnesium stearate / cellulose, microcrystalline / starch, corn / talc / titanium dioxide
Drug Label Author:
Merck Sharp & Dohme Corp.
DEA Schedule:
Non-Scheduled
 [
[