- Physical implications of burn injury
Thermal injury destroys skin, the body’s barrier to the external environment. Skin plays a vital role in thermal regulation, fluid and electrolyte homeostasis, and protection against bacterial infection. Significant heat and protein loss, massive fluid shifts, and infections all commonly occur in patients with severe thermal injuries. Microvascular injury results from local damage by heat and from the release of vasoactive substances from the burned tissue.
- Physiologic implications of burn injury
- In major burns, circulating mediators trigger systemic inflammation, hypermetabolism, and immune suppression. There is also diffuse alteration in the permeability of cell membranes to sodium, resulting in generalized cellular swelling. Therefore, edema occurs in both burned and unburned tissues.
- Cardiovascular effects
- Alterations in microvascular permeability result in a transcapillary fluid flux and significant tissue edema 12 to 24 hours after a thermal injury. Large amounts of water, electrolytes, and proteins are lost into the extravascular space leading to intravascular fluid depletion and hypovolemic shock (burn shock).
- Immediately after a burn injury, the cardiac output is frequently reduced owing to decreased preload and myocardial depression, possibly due to circulating humoral factors. Blood pressure may be normal owing to increased systemic vascular resistance (SVR). The magnitude of these pathophysiologic changes depends on the size and the depth of the burn injury.
- The cardiovascular response 24 to 48 hours after successful resuscitation of a major burn is characterized by an increase in cardiac output and reduced SVR, consistent with the pathophysiology of the systemic inflammatory response syndrome.
- Metabolic effects
- “Ebb phase”: During the first 2 days after severe, acute burns, a hypometabolic state may be seen, accompanied by decreased oxygen consumption, cardiac output, and metabolic rate. This is also known as the “resuscitative phase” of the burn injury, where effective fluid resuscitation is guided by formulas targeted to achieve a urine output of 0.5 to 1 mL/kg/h.
- “Flow phase”: Starting days 2 to 5, there ensues a catecholamine, inflammatory, and hormonally mediated hypermetabolic-hyperdynamic state, which can persist for more than a year. It is frequently accompanied by high cardiac output, decreased SVR, increased oxygen consumption and carbon dioxide production, and protein wasting and catabolism. Treatment during the flow phase should be multifactorial and directed at the various physical and physiologic changes:
- Early skin grafting to aid thermal regulation, electrolyte homeostasis, and protect against bacterial infection.
- Strict temperature regulation with ambient temperature kept within the thermoneutral range to avoid shivering and a further increase in metabolic rate.
- Early nutrition with enteral feeding decreases muscle catabolism and may reduce bacterial translocation through the intestinal mucosa.
- Pharmacologic interventions, such as adrenergic blockade with propranolol, reduce resting energy expenditure, insulin resistance, and muscle-protein catabolism. Other pharmacologic interventions are areas of ongoing interest and research.
- Capillary leakage results in hemoconcentration immediately after injury. Despite apparent adequate fluid resuscitation, the hematocrit level often remains increased during the first 48 hours after injury. Bleeding from wounds and a shortened erythrocyte half-life, however, can result in anemia.
- Microaggregation of the platelets in the skin and smoke-damaged lung and aggressive volume resuscitation result in early thrombocytopenia after major burns. Thrombotic and fibrinolytic mechanisms are activated, and disseminated intravascular coagulation may complicate the course of a massive burn injury. A decrease in antithrombin III, protein C, and protein S levels can increase the thrombogenicity of these patients later in their clinical course and theoretically can cause venous thrombosis and pulmonary embolism.
- Acute renal failure is not uncommon in patients with major burn injury and is associated with high mortality. Decreased renal blood flow secondary to hypovolemia and decreased cardiac output, as well as increased levels of catecholamines, aldosterone, and vasopressin, can contribute to renal failure. Other mechanisms include nephrotoxic effects of drugs, rhabdomyolysis, hemolysis, and sepsis (see Chapter 5).
- Gastrointestinal function is diminished immediately after burn injury, secondary to the development of gastric and intestinal ileus. The stomach should be adequately vented with a nasogastric tube.
- Curling ulcers (mucosal erosion) will occur at variable times after major burns and may lead to gastric hemorrhage or perforation. These ulcers seem to be more common in children than in adults. Therapy consists of antacids, histamine (H2) receptor antagonists, and proton pump inhibitors.
- Other gastrointestinal complications of burns include esophagitis, tracheoesophageal fistula (from prolonged intubation and the presence of a nasogastric tube), hepatic dysfunction, pancreatitis, acalculous cholecystitis, and mesenteric artery thrombosis.
- Infection of burned areas delays healing and prevents successful skin grafting. Bacterial invasion of underlying tissue may result in septicemia. Common organisms involved are staphylococci, β-hemolytic streptococci, and gram-negative rods such as Pseudomonas and Klebsiella species. Local treatment with topical antimicrobials and early skin grafting are important measures to reduce risk of infection.
- Cardiovascular effects
- In electrical burns, current creates thermal energy that destroys tissue, particularly tissues with high resistance such as skin and bone. Exposure to high voltage can result in compartment syndromes, fractures of long bones and the axial spine, myocardial injury, and rhabdomyolysis with subsequent renal injury.
- In chemical burns, the degree of injury depends on the chemical, its concentration, duration of contact, and the penetrability and resistance of the tissues involved. Some substances producing chemical burns, such as phosphorus, are absorbed systemically, producing significant and often life-threatening injury. Hydrofluoric acid exposure will cause severe hypocalcemia and requires close monitoring of serum calcium levels. Subeschar injection of calcium gluconate and emergent wound excision may be indicated. Many chemical agents can be safely and copiously irrigated with water. Chemicals that should not be irrigated include dry lime, elemental metals, and phenol. If the name of the offending chemical agent can be determined, websites, including OSHA, as well as Poison Control provide Safety Data Sheets (formerly known as Material Safety Data Sheets) that provide relevant chemical information and first aid care.
- In major burns, circulating mediators trigger systemic inflammation, hypermetabolism, and immune suppression. There is also diffuse alteration in the permeability of cell membranes to sodium, resulting in generalized cellular swelling. Therefore, edema occurs in both burned and unburned tissues.
- Classification of burn injury
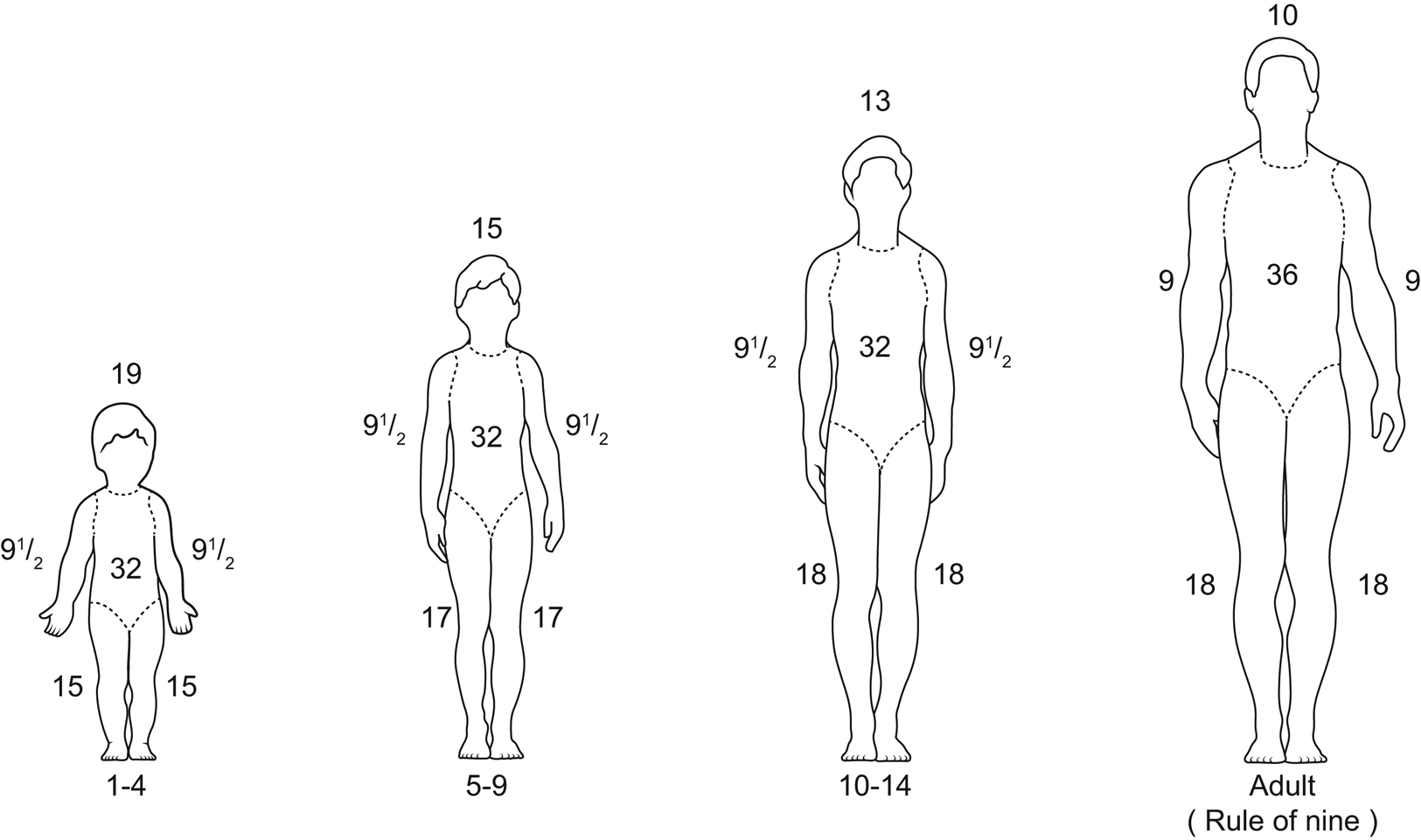
- Burns are classified according to the total body surface area (TBSA) burned, depth of burn, and the presence or absence of inhalational injury. Superficial burns (first degree) should not be included in the TBSA assessment.
- The extent of a burn (TBSA) is calculated by using a Lund-Browder or other burn diagram.
- The rule of nines guides estimation (Figure 35.5).
- Adults: The head and each upper extremity each represent 9% TBSA. The anterior trunk, posterior trunk, and each lower extremity each represent 18% TBSA.
- Infants and children: Because of the different proportions of body surface area relative to patient age, reference must be made to the proper burn chart when calculating percent TBSA to avoid significant errors (Figure 35.5).
- Another practical method to estimate percent TBSA is that the area of the patient’s hand will cover about 1% TBSA.
- The rule of nines guides estimation (Figure 35.5).
- The depth of the burn determines therapy (ie, conservative management vs excision and grafting). Burn depth is difficult to determine visually; however, there are some useful guidelines:
- The area under a partial-thickness burn should have normal or increased sensitivity to pain and temperature and should blanch with pressure.
- A full-thickness burn will be anesthetic and will not blanch with pressure. Commonly, the wound bed has varying depth of burn, so the presence of pain can still be common around areas of full-thickness burn where partial-thickness burns with surviving nerve tissue are also present.
- Initial evaluation of the burn patient
- Airway and breathing
- Brief exposure of the epiglottis or larynx to either dry air at 300 °C or steam at 100 °C leads to massive edema and rapid airway obstruction. Chemical products of combustion such as ammonia, sulfur oxide, and chlorine dissolve in the tracheobronchial tree, forming acids and irritating the mucous membrane of the respiratory tract.
- When airway burns are suspected, early endotracheal intubation should be performed before airway edema occurs. Continued swelling and distortion of the soft tissues may progress at a rapid rate, rendering intubation difficult if not impossible. Upper airway edema usually resolves within the first week and can be reduced by avoiding excessive fluid administration and elevating the head of the bed.
- Circumferential full-thickness burns of the thorax will decrease chest wall compliance, which can lead to hypoxemia and respiratory failure. Emergency escharotomies may be required.
- Ventilation strategies involve avoidance of further respiratory compromise from barotrauma caused by increased alveolar distention and shearing forces. Low tidal volume ventilation is recommended. Bronchospasm is frequently present, and bronchodilator therapy with β2-agonists is warranted. Intraoperatively, minimize retained secretions and cellular debris by attention to body positioning and frequent suctioning.
- Smoke inhalation injury may occur during a fire within a closed space or when heated noxious vapors are inhaled.
- An inhalation injury should be suspected in the presence of burns of the head or neck; singed nasal hairs; swelling of the mucosa of the nose, mouth, lips, or throat; a brassy cough; or carbonaceous sputum. Both the upper airway and pulmonary parenchyma may be affected.
- Chemical products of combustion combine with water in the respiratory tract to form strong acids and alkali, causing bronchospasm, edema, and mucous membrane ulceration. Inhalation of gases such as phosgene and sulfuric acid can damage the alveolar membrane and cause partial or complete airway obstruction. Aldehydes such as acrolein impair ciliary function and damage mucosal surfaces.
- Combustion of polyurethane-containing products (eg, insulation and wall paneling) releases hydrogen cyanide, which causes tissue asphyxia by inhibiting cytochrome oxidase activity. Patients may present with an anion gap metabolic acidosis and an elevated mixed venous PO2. Plasma lactate levels correlate with the cyanide levels. Antidotal treatment of cyanide poisoning involves three strategies: binding of cyanide, induction of methemoglobinemia, and use of sulfur donors.
- Binding cyanide: Hydoxocobalamin/Cyanokit (dose 70 mg/kg IV, maximum dose 5 g) binds CN and forms cyanocobalamin, a less toxic substance.
- Induction of methemoglobinemia provides an alternative binding site for cyanide, rather than the cytochrome electron transport chain complex. Sodium nitrite is the drug of choice (300 mg IV over 5 minutes in 100 mL of 5% dextrose). Amyl nitrate (0.3 mL ampule, crushed and inhaled over 15-30 seconds, repeated every minute) is more technically challenging to administer and induces a much weaker methemoglobinemia, thus should be considered a second-line, temporizing agent. Treatment with amyl nitrite or sodium nitrite is contraindicated in cases of concurrent carbon monoxide toxicity.
- Sulfur donors aid the rhodanese enzyme in detoxifying cyanide and resulting in thiocyanate, which can be renally excreted. Usual dosing is sodium thiosulfate 12.5 g in a 50-mL solution.
- Carbon monoxide binds hemoglobin, displacing oxygen and shifting the oxyhemoglobin curve to the left. Tissue hypoxia ensues.
- All burn-injured patients, especially those burned within a closed space, may have sustained some degree of CO exposure with their thermal injury. Oxygen administration should begin at the scene.
- Because oxyhemoglobin and carboxyhemoglobin absorb light at the same wavelength, conventional pulse oximetry cannot be used as an indicator of CO poisoning. Diagnosis is made on clinical suspicion and the level of arterial or venous carboxyhemoglobin, measured spectrophotometrically with a CO-oximeter.
- The half-life of CO is inversely related to the inspired oxygen concentration (FIO2); it is 5 to 6 hours when breathing room air but 30 to 60 minutes when breathing 100% oxygen.
- Treatment is supportive and consists of supplemental oxygen until the carbon monoxide is eliminated. Hyperbaric oxygen should be considered in comatose patients and in those with severe carbon monoxide poisoning.
- Indirect respiratory injury and pulmonary edema may occur in burn patients without an inhalational injury. Mechanisms involved include the effect of burn wound mediators on the lung, decreased plasma oncotic pressure, and complications of burn therapy.
- Cardiovascular resuscitation
- Fluid replacement consists of crystalloid, usually Ringer lactate, with or without the addition of colloid. Standard protocols for fluid replacement use body weight in kilograms and percent TBSA burned.
- Parkland formula: 4 mL of Ringer lactate per kg per % TBSA burn administered in the first 24 hours. Half of the fluid is given within the first 8 hours from the burn incident, and the remaining over the next 16 hours.
- Brooke formula: 1.5 mL of crystalloid per kg per % TBSA burn per 24 hours plus 0.5 mL of colloid per kg per % TBSA burn per 24 hours plus 2000 mL of 5% dextrose in water per 24 hours.
- Rule of Tens: 10 × % TBSA. The military Joint Trauma System Clinical Practice Guidelines use the Rule of Tens. It is simple to calculate and applies to most adults. The initial fluid rate is calculated as 10 mL × % TBSA, for patients between 40 and 80 kg. If over 80 kg, add 100 mL/h for every 10 kg > 80 kg.
- Half the calculated fluid deficit is administered during the first 8 hours postburn, and the remainder is administered over the next 16 hours. The patient’s daily maintenance fluid requirements are given concurrently.
- The end points of fluid therapy are hemodynamic stability and maintenance of an adequate urine output. In extensive burns, fluid management is adjusted according to appropriate invasive monitors and laboratory studies.
- Fluid replacement consists of crystalloid, usually Ringer lactate, with or without the addition of colloid. Standard protocols for fluid replacement use body weight in kilograms and percent TBSA burned.
- Airway and breathing
- Management of the burn wound
- Early excision and grafting of burned areas is a widely accepted procedure and appear to decrease mortality and hospital length of stay. Patients may be brought to the operating room in the acute phase of injury, with hemodynamic instability and respiratory dysfunction. Special emphasis should be placed on correcting acid-base and electrolyte disturbances, temperature normalization, and treatment of anemia and coagulopathy. Blood loss during wound excision and grafting can be massive. Adequate colloid and blood products should be ordered in advance. IV access should be adequate for resuscitation.
- Topical agents are used to minimize the colonization of healing wounds. Burns should be kept moist, and many centers use petroleum-based agents with or without antibiotics (such as Bacitracin) and nonadherent, petroleum-impregnated dressings (such as Xeroform gauze). Other topical agents and their side effects include:
- Silver nitrate may cause hyponatremia or, rarely, methemoglobinemia.
- Silver sulfadiazine may cause leukopenia, which is reversible upon discontinuing the drug.
- The incidence of sepsis may be reduced by using temporary biologic dressings, either allografts (cadaver skin or amnion) or xenografts (porcine). Artificial skin (eg, Integra) bioengineered from collagen and cultured epidermis can be used when a conventional autograft is not available.
- The use of systemic antibiotics is limited to treatment of documented systemic infection (as opposed to colonization), and they are also used as prophylaxis before surgical procedures.
- Anesthetic considerations
- Burn-injured patients may also suffer from nonthermal traumatic injuries and should initially be assessed as a trauma patient (see Sections I.A to I.E). The patient’s age, preexisting disease, and the extent of burn injury provide an index of the patient’s likely physiologic condition. Altered pharmacokinetics, drug tolerance, difficult IV access, and anatomic derangements of the airway (neck scar or mouth contracture) are primary considerations.
- Airway. Obtaining an adequate mask fit may be difficult because of edema in the early phases of burn injury or because of scars and contractures later on. The same processes can render endotracheal intubation extremely difficult in burn patients.
- Monitoring and IV access (Table 35.2)
- Often IV access will still be in place from the initial resuscitation. Large-bore IVs are mandatory to allow for massive fluid replacement.
Table 35-2 Intraoperative Monitoring Challenges in Patients With Major Burn Injury
Monitor Possible Challenges of Burn Injury Possible Solutions Electrocardiography (ECG) ECG electrodes may not adhere Consider needle electrodes or stapling of electrodes Noninvasive blood pressure monitor Edema or extensive burns in the extremities may limit use of cuff Consider alternative location, use dressing or gauze under cuff
Consider invasive monitoringInvasive blood pressure monitor Hypothermia, hypovolemia, initial decreased cardiac output, vasoconstriction Consider alternative location, trend values, confirm with noninvasive blood pressure Pulse oximetry Extensive burn injury may limit placement
Carboxyhemoglobin will falsely elevate oxygen saturation readingConsider alternative location, trend values, assess partial pressure of oxygen by arterial blood gas (ABG) - In massive burns, ECG electrodes may be placed directly on debrided tissue. Alternatively, needle electrodes can be used.
- Arterial lines are indispensable for continuous blood pressure monitoring and frequent blood sampling. The cannulation site will depend on the availability of unburned areas. If all appropriate sites are burned, the line may have to be placed through the burn wound after the area has been prepared in a sterile fashion.
- CVP lines are useful both for monitoring central pressures and for central access for drug infusions.
- A pulmonary artery catheter may be required for management of patients with myocardial dysfunction, persistent oliguria or hypotension, or sepsis.
- Often IV access will still be in place from the initial resuscitation. Large-bore IVs are mandatory to allow for massive fluid replacement.
- Muscle relaxants. Extrajunctional nicotinic receptors proliferate in muscle at the burn site and at sites distant from the burn injury. Succinylcholine is only safe in the immediate 24 hours after thermal injury. An increase in acetylcholine receptors is usually associated with resistance to nondepolarizing neuromuscular-blocking agents, increased sensitivity to depolarizing muscle relaxants, and life-threatening hyperkalemia when succinylcholine is used.
- Anesthetics
- There is no single preferred agent or combination of agents; however, ketamine and etomidate may have advantages in patients with a tenuous hemodynamic status.
- These patients may have greatly increased opioid requirements owing to tolerance, hypermetabolic physiology, and an increase in the apparent volume of distribution for drugs. It is important to provide adequate analgesia, which may necessitate massive doses of opiates.
- Temperature regulation. The most comfortable body temperature for a burn patient is about 100 °F (38 °C). In the burn intensive care unit, patients are cared for in warmed, humidified rooms. Every effort should be made to maintain normothermia during transport and surgery. The operating room, IV fluids, and blood products should be warmed and inspired gases heated and humidified. Pediatric patients should be placed under a radiant heat source and on a warming blanket whenever possible.
- Immunosuppression. The immune system is suppressed for weeks to months after burn injury, and the wound itself serves as an excellent medium for bacterial growth. Every attempt should be made to practice aseptic technique when handling patients, suctioning airways, and inserting intravascular lines.
- Postanesthetic care. It is important to maintain normothermia while transporting patients back to the intensive care unit because shivering results in vasoconstriction and could contribute to graft loss. Supplemental oxygen should be given until patients are fully recovered from anesthesia. Severe pain is common, and patient responses vary, necessitating individual titration of analgesics and frequent reassessment of the effect.