Author(s): Lukas H.Matern, Marvin G.Chang
The decision to initiate transfusion is complex. There are many risks to transfusion (see Section VIII), and transfusion is ultimately indicated when the potential benefits outweigh these risks. In most civilian settings, transfusion therapy utilizes blood components rather than whole blood. The benefits of blood component transfusion stem from the correction of conditions that result in decreased production; increased utilization, destruction, or loss; or dysfunction of a specific blood component (usually red blood cells, platelets, or coagulation factors). Although many attempts at creating universal transfusion protocols have been introduced, few have successfully demonstrated definitive thresholds or clear superiority. The aim of this chapter is to provide the background to help clinicians make these decisions on an individual basis. A condensed summary of approaches to perioperative transfusion therapy and decision-making may be found in Table 36.1.
Table 36-1 Summary of Perioperative Transfusion Considerations
| ||||
- Assessing red cell mass and oxygen-carrying ability
- The decision to transfuse red blood cells (also called erythrocytes, red cells, or RBCs). Most evidence suggests that higher red cell transfusion thresholds (eg, 10-12 g/dL) do not confer a mortality benefit and may even be harmful. Nonetheless, maintaining adequate red cell mass is essential for adequate oxygen-carrying capacity to the tissues, and it has been shown that perioperative anemia may increase the risk of acute kidney injury, acute coronary or cerebrovascular events, and prolonged postoperative hospital stays. The decision to transfuse, therefore, must be weighed carefully based on a variety of patient and situational factors rather than rigid guidelines.
- Patient factors. The main reason for transfusing red cells is to maintain the oxygen-carrying capacity to the tissues, a primary determinant of which is the hemoglobin (Hb) level.
- Healthy individuals or individuals with chronic anemia can usually tolerate lower Hb levels of 6.5 to 8 g/dL, assuming normal intravascular volume. The use of a “restrictive” transfusion practice, aiming for Hb of 7 to 9 g/dL, is generally safe and may reduce the risk of death compared with transfusing toward a “liberal” Hb goal of 10 to 12 g/dL. The reasons for reduced mortality in the restrictively transfused group have not been clearly delineated, but the effects of allogeneic transfusion on diminished immune function have been substantiated in animal and human studies (see Section VIII.D).
- For patients with coronary artery disease (CAD), the risk of myocardial ischemia due to anemia has resulted in most practitioners transfusing to a higher (9-10 g/dL) Hb target. As the incidence of CAD rises substantially with age, many practitioners also opt to use a higher transfusion threshold in the elderly. Studies to support this practice, however, are lacking, and those that exist have produced contradictory results. For example, a study of medical patients with acute coronary syndromes also found a higher mortality rate attributable to red cell transfusion in otherwise stable patients with a hematocrit (Hct) greater than 25%.
- Situational factors
- Intraoperative red cell transfusion depends on red cell loss. This can be roughly estimated by measuring blood in suction canisters, weighing sponges, and checking blood loss in the drapes. In the perioperative period, suspected or confirmed hemorrhage remains the most common indication for transfusion. During periods of ongoing blood loss, transfusion may be indicated even with a Hb > 10 g/dL if bleeding is brisk enough that the clinician expects a substantial drop in Hb without treatment.
- If a patient is anemic preoperatively, the etiology should be clarified. It may be secondary to decreased production (marrow suppression or nutritional deficiencies), increased loss (hemorrhage), or destruction (hemolysis). Such an assessment may then be used to guide perioperative therapy.
- Estimating blood volumes (BVs)
- Estimated allowable blood loss (EABL) can be calculated as follows, using either Hcts (as shown) or Hbs:
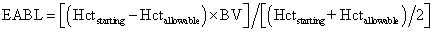
- BV in an adult is approximately 7% of lean body mass. This may be calculated as approximately 70 mL/kg of body weight in an average adult man and approximately 65 mL/kg of body weight in an average adult woman (see Chapter 33 for pediatric considerations). Obese patients have a lower relative lean body mass and a lower relative BV; thus, the greater the degree of obesity, the lower the BV estimate relative to total body weight (TBW). For example, a patient with a body mass index (BMI) of 40 may have an estimated BV of 53 mL/kg TBW, whereas patients with a BMI of 70 may have an estimated BV of 40 mL/kg TBW.
- The volume of blood to transfuse can be calculated as follows:
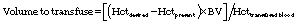
- One unit of packed red blood cells (PRBCs) has a Hct of 70% to 85% when stored with the standard Adsol preservative.
- Estimated allowable blood loss (EABL) can be calculated as follows, using either Hcts (as shown) or Hbs:
- Thrombocytopenia is due to either decreased bone marrow production (eg, chemotherapy, tumor infiltration, or alcoholism) or increased utilization or destruction (eg, trauma or surgery creating a large wound, hypersplenism, idiopathic thrombocytopenia purpura [ITP], disseminated intravascular coagulation [DIC], or drug effects). It also results from dilution and loss associated with massive transfusion (see Section IX.A.1). Spontaneous bleeding is unusual with platelet counts above 20,000/mm3. Platelet counts above 50,000/mm3 are preferable for surgical hemostasis. However, as in the case of red cell administration, the decision to transfuse must be made based upon clinical factors rather than platelet count alone. For example, patients with ITP have a high rate of platelet turnover and will often exhibit normal coagulation despite a low platelet count. In addition, as this form of thrombocytopenia is primarily due to platelet destruction, patients with ITP will not respond adequately to platelet transfusion. Instead, treatments should be aimed at halting the underlying destructive process.
- Coagulopathy. Bleeding associated with documented factor deficiencies or prolonged clotting studies (prothrombin time [PT] and partial thromboplastin time [PTT]) mandates replacement therapy to maintain normal coagulation function. See Sections II and IX for a discussion of coagulopathy.