Key Clinical Updates in Hyperthyroidism (Thyrotoxicosis) The medication digoxin is a second-line agent for ventricular rate control in thyrotoxicosis-induced atrial fibrillation. It is reduced as hyperthyroidism is corrected. Serum digoxin levels should be kept below 1.2 ng/mL, since higher levels are associated with increased mortality. |
| ESSENTIALS OF DIAGNOSIS | ||
|
General Considerations
The term "thyrotoxicosis" refers to the clinical manifestations associated with elevated serum levels of T4 or T3 that are excessive for the individual (hyperthyroidism).
A. Graves Disease
Graves disease is the most common cause of thyrotoxicosis. It is an autoimmune disorder, characterized by an increase in synthesis and release of thyroid hormones. Autoantibodies, known as thyroid-stimulating immunoglobulins (TSI) or thyrotropin receptor antibodies (TRAb), bind to the TSH receptors in the thyroid cell membranes and stimulate the gland to overproduce thyroid hormones. The presence of these antibodies distinguishes Graves disease from autoimmune chronic lymphocytic (Hashimoto) thyroiditis. Both conditions usually have present serum antithyroid antibodies (TPO Ab or Tg Ab or both).
Graves disease is more common in women than in men (8:1). Its usual onset is between the ages of 20 and 40 years. It may be accompanied by infiltrative ophthalmopathy (Graves exophthalmos) and, less commonly, by infiltrative dermopathy (pretibial myxedemaeFigure 28-5). The thymus gland is typically enlarged and serum ANA levels are usually elevated. Many patients with Graves disease have a family history of either Graves disease or Hashimoto autoimmune thyroiditis. Histocompatibility studies have shown an association with group HLA-B8 and HLA-DR3.
eFigure 28-5. Pretibial Myxedema
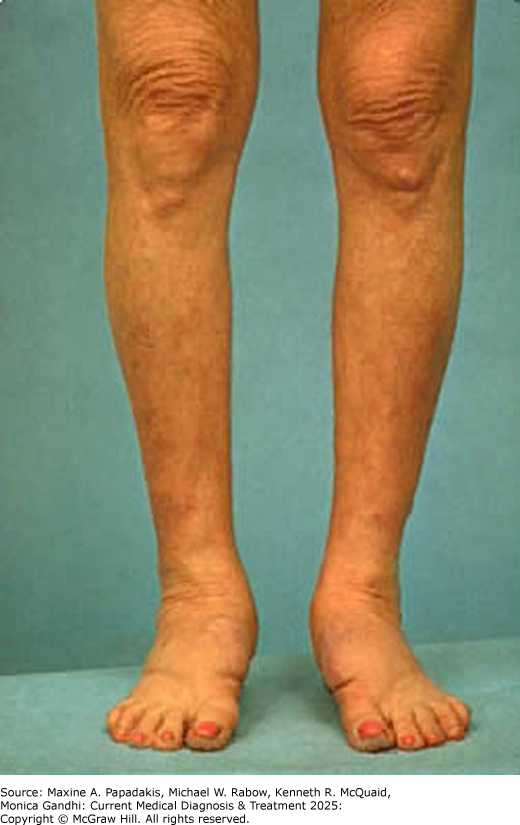
Pretibial myxedema. (Reproduced with permission from Carl Grunfeld, MD)
Viral infections, including infections with SARS-CoV-2, have been reported to precipitate Graves disease. Vaccinations against SARS-CoV-2 also have triggered de novo Graves disease as well as relapses 4-30 days after infection or vaccination.
Patients with Graves disease have an increased risk of other systemic autoimmune disorders, including Sjogren syndrome, celiac disease, pernicious anemia, Addison disease, alopecia areata, vitiligo, type 1 diabetes mellitus, hypoparathyroidism, myasthenia gravis, and cardiomyopathy.
B. Toxic Multinodular Goiter and Thyroid Nodules
Autonomous hyperfunctioning thyroid nodules that produce hyperthyroidism are known as toxic multinodular goiter (Plummer disease). They are more prevalent among older adults and in iodine-deficient regions. A single hyperfunctioning nodule can also produce hyperthyroidism. Activating TSH receptor mutations are responsible for some toxic nodules. Toxic multinodular goiter and Graves disease may sometimes coexist in the same gland (Marine-Lenhart syndrome). Thyroid cancer is found in 5% of patients with toxic multinodular goiter.
C. Autoimmune (Postpartum or Silent) Thyroiditis and Subacute Thyroiditis
These conditions cause thyroid inflammation with release of stored hormone. They all produce a variable triphasic course: variable hyperthyroidism is followed by transient euthyroidism and progression to hypothyroidism (see Thyroiditis, above).
Silent thyroiditis is also known as subacute lymphocytic thyroiditis or "hashitoxicosis." It can occur spontaneously; women are affected four times more frequently than men. About 50% have antithyroid antibodies. Silent thyroiditis can also be caused by chemotherapeutic agents (such as tyrosine kinase inhibitors; denileukin diftitox; alemtuzumab; interferon-alpha; interleukin-2; and immune checkpoint inhibitors). Other drugs can cause silent thyroiditis, including lithium and amiodarone. In those with spontaneous silent thyroiditis, about 10-20% remain hypothyroid after 1 year. There is a recurrence rate of 5-10%; this rate is higher in Japan.
Postpartum thyroiditis refers to autoimmune thyroiditis that occurs in the first 12 months postpartum and occasionally after miscarriages. See Thyroiditis, above. About 22% of affected women experience hyperthyroidism followed by hypothyroidism, whereas 30% of such women have isolated thyrotoxicosis and 48% have isolated hypothyroidism. The thyrotoxic phase typically occurs 2-6 weeks postpartum and lasts 2-3 months. Over 80% have antithyroid antibodies. Most women progress to a hypothyroid phase that usually lasts a few months but that can be permanent.
Subacute thyroiditis is also known as "de Quervain" or "granulomatous" thyroiditis. It is typically caused by various viral infections. Women are affected four times more frequently than men. Patients typically experience a viral upper respiratory infection and develop an enlarged and extremely painful thyroid. About 50% of affected patients experience a symptomatic thyrotoxic phase that lasts 3-6 weeks. It is important to differentiate subacute thyroiditis from infectious (suppurative bacterial) thyroiditis. About 10% remain hypothyroid after 1 year. The recurrence rate is 1-4%.
D. Medication-Induced Hyperthyroidism
1. Amiodarone-Induced Thyrotoxicosis (Ait)
Amiodarone causes thyrotoxicosis in about 5% of patients in the United States, with a higher incidence in iodine-deficient geographic areas. Amiodarone is 37% iodine by weight and its metabolites have a half-life of about 100 days. In the short term, amiodarone normally increases serum TSH (without hypothyroidism), though usually not over 20 mIU/L. Serum T4 and FT4 rise about 40% and may become elevated in clinically euthyroid patients, while serum T3 levels typically decline. After 3 months, the serum TSH usually normalizes. Thyroid function tests (TSH, FT4, T3) should be checked before starting amiodarone, after 3-6 months of therapy, and thereafter at least every 6 months (sooner if clinically warranted). Due to early short-term changes, it is best to not check thyroid function tests during the first 3 months of therapy.
Amiodarone-induced thyrotoxicosis (AIT) can occur at any time during treatment and may develop several months after treatment discontinuation. It is diagnosed when serum TSH levels are suppressed and serum T3 or FT3 levels are high or high-normal. Amiodarone is the leading cause for thyrotoxic crisis ("thyroid storm"); however, the manifestations can be missed since amiodarone tends to cause bradycardia. Type 1 AIT is caused by the active production of excessive thyroid hormone and typically occurs within 2-6 months after starting amiodarone. Type 2 AIT is caused by the passive release of stored thyroid hormone and occurs an average of 30 months after starting amiodarone. A mixed/indeterminate type of AIT is caused by both processes occurring simultaneously.
2. Iodine-Induced Hyperthyroidism (Basedow Disease)
The recommended iodine intake for nonpregnant adults is 150 mcg/day. Higher iodine intake can precipitate hyperthyroidism in patients with nodular goiters, autonomous thyroid nodules, or asymptomatic Graves disease, and less commonly in patients with no detectable underlying thyroid disorder. Common sources of excess iodine include intravenous oral potassium iodine supplements, certain foods (eg, kelp, nori), topical iodinated antiseptics (eg, povidone iodine), and medications (eg, amiodarone or potassium iodide). Intravenous iodinated radiocontrast dye can rarely induce a painful, destructive subacute thyroiditis, similar to type 2 amiodarone-induced thyrotoxicosis.
3. Tyrosine Kinase Inhibitors
Silent autoimmune thyroiditis that releases stored thyroid hormone, resulting in hyperthyroidism, develops in about 3% of patients receiving chemotherapy with tyrosine kinase inhibitors (eg, axitinib, sorafenib, sunitinib). While such hyperthyroidism may be subclinical, thyrotoxic crisis has been reported. Hypothyroidism usually follows hyperthyroidism and occurs in 19% of patients taking these drugs.
4. Immune Checkpoint Inhibitor Cancer Therapy
Immune checkpoint inhibitor therapy directed against either PD-1/PD-L1 or CTLA-4/B7-1/B7-2 frequently precipitates autoimmune adverse reactions. Thyroid autoimmunity commonly causes thyroiditis, hypothyroidism (primary or secondary), or hyperthyroidism from either passive release of thyroid hormone or active production of thyroid hormone (Graves disease).
E. Pregnancy, Hcg-Secreting Trophoblastic Tumors, and Testicular Choriocarcinoma
Human chorionic gonadotropin (hCG) can bind to the thyroid's TSH receptors. Very high levels of serum hCG, particularly during the first 4 months of pregnancy may cause sufficient receptor activation to cause gestational thyrotoxicosis. About 20% of pregnant women have a low serum TSH during pregnancy, but only 1% of such women have clinical hyperthyroidism that requires treatment. Pregnant women are more likely to have hCG-induced thyrotoxicosis if they have high levels of serum asialo-hCG, a subfraction of hCG that has a greater affinity for TSH receptors. Such women are also more likely to suffer from hyperemesis gravidarum. This condition must be distinguished from true Graves disease in pregnancy, which usually predates conception and may be associated with high serum levels of TSI and antithyroid antibodies or with exophthalmos.
F. Rare Causes of Hyperthyroidism
Thyrotoxicosis factitia is due to intentional or accidental ingestion of excessive amounts of exogenous thyroid hormone. Struma ovarii is thyroid tissue contained in about 3% of ovarian dermoid tumors and teratomas. Such ectopic thyroid tissue can produce excess thyroid hormone from thyroid nodules or as a concomitant source of thyroid hormone excess with Graves disease. Pituitary TSH hypersecretion by a pituitary thyrotroph tumor or hyperplasia can rarely cause hyperthyroidism; serum TSH is elevated or inappropriately normal in the presence of true thyrotoxicosis. Pituitary hyperplasia may be detected on an MRI as a pituitary enlargement without a discrete adenoma being visible. This condition appears to be due to a diminished feedback effect of T4 upon the pituitary. Some cases are familial. Metastatic functioning thyroid carcinoma can cause hyperthyroidism in patients with a heavy tumor burden. Recombinant human thyroid-stimulating hormone (rhTSH) can rarely induce hyperthyroidism when it is given prior to RAI therapy or scanning for metastatic differentiated thyroid cancer.
High levels of hCG can also cause thyrotoxicosis in some cases of pregnancies with gestational trophoblastic disease (molar pregnancy, choriocarcinoma). Some such pregnancies have produced thyrotoxic crisis. Men have developed hyperthyroidism from high levels of serum hCG secreted by a testicular choriocarcinoma.
Clinical Findings
A. Symptoms and Signs
Thyrotoxicosis can produce nervousness, restlessness, heat intolerance, increased sweating, palpitations, pruritus, fatigue, muscle weakness, muscle cramps, frequent bowel movements, weight change (usually loss), or menstrual irregularities. There may be fine resting finger tremors, moist warm skin, fever, hyperreflexia, fine hair, and onycholysis (eFigure 28-6). Angina or atrial fibrillation may also be present, sometimes in the absence of other thyrotoxic symptoms (apathetic hyperthyroidism). Women with postpartum thyroiditis are often asymptomatic or experience only minor symptoms, such as palpitations, heat intolerance, and irritability. Chronic thyrotoxicosis may cause osteoporosis. Even subclinical hyperthyroidism (suppressed serum TSH with normal FT4) may increase the risk of nonvertebral fractures. Tetany is a rare presenting symptom.
eFigure 28-6. Onycholysis (Separation of the Nail from its Bed) in Graves Disease Usually Resolves Spontaneously as the Patient Improves
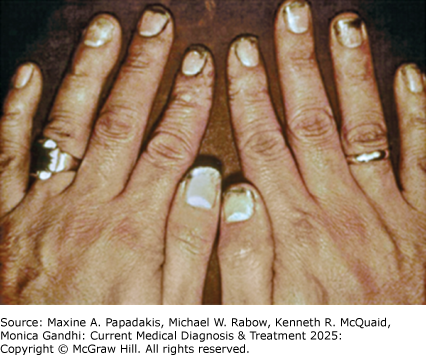
Onycholysis (separation of the nail from its bed) in Graves disease usually resolves spontaneously as the patient improves. (Reproduced, with permission, from Gardner DG, Shoback D [editors]. Basic & Clinical Endocrinology, 10th ed. New York, NY: McGraw-Hill; 2018.)
Patients with Graves disease usually have a diffusely enlarged thyroid that is frequently asymmetric and often accompanied by a bruit. However, there may be no palpable thyroid enlargement. The thyroid gland in painful subacute thyroiditis is usually moderately enlarged and tender. There is often dysphagia and pain that can radiate to the jaw or ear. With toxic multinodular goiter, there are usually palpable nodules. Patients with silent thyroiditis or postpartum thyroiditis have either no palpable goiter or a small, nontender goiter.
Cardiopulmonary manifestations of thyrotoxicosis commonly include a forceful heartbeat, premature atrial contractions, and sinus tachycardia. Patients often have exertional dyspnea. Atrial fibrillation or atrial tachycardia occurs in about 8% of patients with thyrotoxicosis, more commonly in men, older adults, and those with ischemic or valvular heart disease. The ventricular response from the atrial fibrillation may be difficult to control. Thyrotoxicosis can cause a thyrotoxic cardiomyopathy, and the onset of atrial fibrillation can precipitate HF. Echocardiogram reveals pulmonary artery hypertension in about 40% of hyperthyroid patients. Even "subclinical hyperthyroidism" increases the risk for atrial fibrillation and overall mortality. Hemodynamic abnormalities and pulmonary hypertension are reversible with restoration of euthyroidism.
Thyrotoxic crisis or "thyroid storm" is an extreme form of severe thyrotoxicosis and an immediate threat to life. The most common manifestations are cardiac (HF, severe sinus tachycardia [60%], ventricular fibrillation [13%], MI, and cardiogenic shock), agitation or delirium (63%), high fever, vomiting, diarrhea, dehydration, and hepatic impairment (52%). The presence of thyrotoxic crisis can be assessed by the Burch-Wartofsky score that is based on clinical manifestations: a score less than 25 excludes thyrotoxic crisis, while a score of 25-45 indicates incipient crisis, and a score greater than 45 indicates probable thyrotoxic crisis.
Eye manifestations that occur with hyperthyroidism are discussed in Thyroid Eye Disease, below.
Graves dermopathy (pretibial myxedema) occurs in about 3% of patients with Graves disease. It usually affects the pretibial region but can also affect the dorsal forearms and wrists and dorsum of the feet. It is more common in patients with high levels of serum TSI and severe Graves ophthalmopathy. Glycosaminoglycans accumulation and lymphoid infiltration occur in affected skin, which becomes erythematous with a thickened, rough texture (eFigure 28-5).
Thyroid acropachy is a rare skeletal manifestation of Graves disease. It presents with digital clubbing, swelling of fingers and toes, and radiographic findings of periostitis involving phalangeal and metacarpal bones. Extremity skin can become very thickened, resembling elephantiasis. Thyroid acropachy is ordinarily associated with ophthalmopathy and thyroid dermopathy. Most affected patients are smokers. The presence of thyroid acropachy is an indication of the severity of the autoimmunity; most patients have high serum titers of TSI.
Clinical hyperthyroidism during pregnancy has a prevalence of about 0.2%. It may commence before conception or emerge during pregnancy, particularly the first trimester. Pregnancy can have a beneficial effect on the thyrotoxicosis of Graves disease, with decreasing antibody titers and decreasing serum T4 levels as the pregnancy advances; about 30% of affected women experience a remission by late in the second trimester. Undiagnosed or undertreated hyperthyroidism carries an increased risk of miscarriage, preeclampsia-eclampsia, preterm delivery, abruptio placenta, maternal HF, and thyrotoxic crisis (thyroid storm). Such thyrotoxic crisis can be precipitated by trauma, infection, surgery, or delivery and confers a fetal/maternal mortality rate of about 25%.
Thyroid-stimulating immunoglobulin (TSI, TSHrAb) crosses the placenta. If maternal serum TSI levels reach greater than 500% in the third trimester, the risk of transient neonatal Graves disease in the newborn is increased. Such thyrotoxic newborns have an increased risk of intrauterine growth retardation and prematurity.
Hypokalemic periodic paralysis occurs in about 15% of Asian or American Indian men with thyrotoxicosis and is 30 times more common in men than women. It is marked by sudden symmetric flaccid paralysis, along with hypokalemia and hypophosphatemia, that occurs during hyperthyroidism (often after intravenous dextrose, oral carbohydrates, or vigorous exercise) despite few, if any, of the classic signs of thyrotoxicosis. Attacks last 7-72 hours.
B. Laboratory Findings
Serum FT4, T3, FT3, and T4, thyroid resin uptake, and FT4 index are all usually increased. Sometimes the FT4 level may be normal but with an elevated serum T3 (T3 toxicosis). The severity of the elevation of serum FT4 and FT3 levels does not always correlate with the severity of thyrotoxic manifestations; patients with thyrotoxic crisis tend to have serum thyroid levels that are not significantly higher than those with less pronounced symptoms. Serum T3 can be misleadingly elevated when blood is collected in tubes using a gel barrier, which causes certain immunoassays (eg, Immulite but not AxSYM analyzers) to report falsely elevated serum total T3 levels in 24% of normal patients. Serum T4 or T3 can be elevated in other nonthyroidal conditions (Table 28-4. Factors that Can Cause Misleading Laboratory Tests for Hyperthyroidism).
Table 28-4. Factors that can cause misleading laboratory tests for hyperthyroidism.High Serum T4 or T3 | Low Serum TSH |
|---|---|
Laboratory error Collection vial contains gel barrier for T3 Acute psychiatric problems (30%) Acute or chronic active hepatitis, primary biliary cirrhosis AIDS (increased TBG) Autoimmunity Euthyroid sick Familial thyroid-binding abnormalities Familial resistance to thyroid (Refetoff syndrome) Pregnancy: morning sickness, hyperemesis gravidarum Drugs Amiodarone Amphetamines Biotin supplements (certain assays) Capecitabine Clofibrate Estrogens (oral) Heparin Heroin, methadone Perphenazine Tamoxifen Thyroid hormone therapy (excessive or factitious) | Laboratory error African descent (3-4%) Autonomous thyroid or thyroid nodule Corticosteroids (short-term use) Drugs Amphetamines Biotin supplements (certain assays) Calcium channel blockers (nifedipine, verapamil) Dobutamine Dopamine Dopamine agonists Glucocorticoids Metformin Somatostatin analogs Thyroid hormone Elderly euthyroid hCG-secreting trophoblastic tumors Hypopituitarism Nonthyroidal illness (severe) Pregnancy (especially with morning sickness) Suppression after recent therapy for hyperthyroidism TSH variants not detected by commercial assays |
hCG, human chorionic gonadotropin; T4, levothyroxine; T3, triiodothyronine; TBG, thyroid-binding globulin.
Serum TSH is suppressed in hyperthyroidism (except in the very rare cases of pituitary inappropriate secretion of thyrotropin). Serum TSH may be misleadingly low in other nonthyroidal conditions (Table 28-4. Factors that Can Cause Misleading Laboratory Tests for Hyperthyroidism). The term "subclinical hyperthyroidism" is used to describe individuals with a low serum TSH but normal serum levels of FT4 and T3; in such patients, the overall prevalence of symptomatic hyperthyroidism is 0.7-1.8% in iodine-sufficient patients and 2-15% in patients with iodine deficiency. About two-thirds of patients with subclinical hyperthyroidism have serum TSH levels of 0.1-0.4 mIU/L (mild subclinical hyperthyroidism), while the remainder have serum TSH levels below 0.1 mIU/L (severe subclinical hyperthyroidism).
Hyperthyroidism can cause hypercalcemia, increased liver enzymes, increased alkaline phosphatase, anemia, and neutropenia. Hyperthyroidism also increases urinary magnesium excretion, which can lead to hypomagnesemia, functional hypoparathyroidism with hypocalcemia, and tetany (rarely). Hypokalemia and hypophosphatemia occur in thyrotoxic periodic paralysis.
Problems of diagnosis occur in patients with acute psychiatric disorders; about 30% of these patients have elevated serum T4 levels without clinical thyrotoxicosis. The TSH is not usually suppressed, distinguishing psychiatric disorder from true hyperthyroidism. T4 levels return to normal gradually.
In Graves disease, serum thyroid-stimulating immunoglobulin (TSI, TSHrAb) is usually detectable (65%). Very high serum TSI levels predispose to Graves ophthalmopathy. Serum TSH levels above 350 mIU/L can potentially cause false-positive TSI results. TPO Ab or Tg Ab are usually elevated but are nonspecific. Serum ANA are also usually elevated without any evidence of SLE or other rheumatologic disease.
With painful subacute thyroiditis, patients often have an increased WBC, ESR, and CRP. About 25% have antithyroid antibodies (usually in low titer) and serum TSI (TSHrAb) levels are normal. Patients with iodine-induced hyperthyroidism have undetectable serum TSI (or TSHrAb), no serum TPO Ab, and an elevated urinary iodine concentration. In thyrotoxicosis factitia, serum thyroglobulin levels are low, distinguishing it from other causes of hyperthyroidism.
With hyperthyroidism during pregnancy, women have an elevated serum total T4 and FT4 while the TSH is suppressed. An apparent lack of full TSH suppression in hyperthyroidism can be seen due to misidentification of hCG as TSH in certain assays. The serum FT4 assay is difficult to interpret in pregnancy. Although the serum T4 is elevated in most pregnant women, values over 20 mcg/dL (257 nmol/L) are encountered only in hyperthyroidism. The T3 resin uptake, which is low in normal pregnancy because of high thyroxine-binding globulin (TBG) concentration, is normal or high in thyrotoxic persons.
Since high levels of T4 and FT4 are normally seen in patients taking amiodarone, a suppressed TSH must be present along with a greatly elevated T4 (greater than 20 mcg/dL [257 nmol/L]) or T3 (greater than 200 ng/dL [3.1 nmol/L]) in order to diagnose hyperthyroidism. In type 1 amiodarone-induced thyrotoxicosis, the presence of proptosis and serum TSI (TSHrAb) is diagnostic. In type 2 amiodarone-induced thyrotoxicosis, serum levels of interleukin-6 (IL-6) are usually quite elevated.
C. Radioisotope Uptake and Imaging
Note:All radioisotope testing is contraindicated during pregnancy or breastfeeding.
Radioiodine (123 I) scanning can be helpful in some situations to determine the cause of hyperthyroidism but is unnecessary for diagnosis in patients with obvious Graves disease who have elevated serum TSI or associated Graves ophthalmopathy. A high thyroid RAI uptake is seen in Graves disease and toxic nodular goiter. The isotope is administered orally and thyroidal RAI uptake is determined at about 4-6 hours and again at 24 hours, when a scan is also performed. Normal RAI uptake is 3-16% at 4-6 hours and 8-35% at 24 hours; however, normal ranges vary by region. A low 123 I RAI uptake is characteristic of iodine-induced hyperthyroidism and thyroiditis (subacute, silent, or postpartum), distinguishing them from Graves disease. Patients with type 1 amiodarone-induced thyrotoxicosis have RAI uptake that is usually detectable, while in type 2 amiodarone-induced thyrotoxicosis, thyroid RAI uptake is usually below 3%. Ideally, the RAI scan should include the pelvis to screen for concomitant struma ovarii (rare).
Misleadingly high thyroid RAI uptake has been reported in patients with the following conditions: kidney disease, iodine deficiency, hypochloremia, recovery phase from subacute or silent thyroiditis, lithium carbonate therapy, estrogen therapy, phenothiazine therapy, some congenital disorders of thyroid hormone synthesis, and following withdrawal of thiourea therapy (rebound phenomenon).
Technetium (Tc-99m) pertechnetate thyroid uptake is increased or normal with Graves disease, whereas those with thyrotoxicosis from thyroiditis (silent, subacute, postpartum) have reduced uptake. It is not helpful to distinguish type I from type II amiodarone-induced thyrotoxicosis, since uptake is typically reduced in both.
99mTc-sestamibi (MIBI) scanning usually shows increased uptake with type 1 amiodarone-induced thyrotoxicosis (AIT), decreased uptake in type 2 AIT, and intermediate uptake in mixed AIT.
D. Other Imaging
Thyroid ultrasound can be helpful in hyperthyroid patients with palpable thyroid nodules. Thyroid ultrasound shows a variably heterogeneous, hypoechoic gland in thyroiditis. Color flow Doppler sonography is helpful to distinguish type 1 amiodarone-induced thyrotoxicosis (enlarged gland with normal to increased blood flow velocity and vascularity) from type 2 amiodarone-induced thyrotoxicosis (distorted gland without increased vascularity).
MRI and CT scanning of the orbits are the imaging methods of choice to visualize Graves ophthalmopathy affecting the extraocular muscles. Imaging is required only in severe or unilateral cases or in euthyroid exophthalmos that must be distinguished from orbital pseudotumor, tumors, and other lesion. Chest, CT in Graves disease often detects an enlarged thymus gland.
Differential Diagnosis
True thyrotoxicosis must be distinguished from those conditions that elevate serum T4 and T3 or suppress serum TSH without affecting clinical status (see Table 28-4. Factors that Can Cause Misleading Laboratory Tests for Hyperthyroidism). Biotin supplements can cause a false elevation in free T4 and total T3 and a false suppression of TSH in some assays that use a biotin-streptavidin fluorescent detection system. Biotin can also cause false-positives in some assays for thyrotropin receptor antibodies (TSHrAb), resulting in a misdiagnosis of Graves disease. Serum TSH is commonly suppressed in early pregnancy and only about 10% of pregnant women with a low TSH have clinical hyperthyroidism.
States of hypermetabolism without thyrotoxicosis-notably severe anemia, leukemia, polycythemia, cancer, and pheochromocytoma-rarely cause confusion. Acromegaly may also produce tachycardia, sweating, and thyroid enlargement.
The differential diagnosis for thyroid-associated ophthalmopathy includes an orbital tumor (eg, lymphoma) or pseudotumor. Ocular myasthenia gravis is another autoimmune condition that occurs more commonly in Graves disease but is usually mild, often with unilateral eye involvement. Acetylcholinesterase receptor antibody (AChR Ab) levels are elevated in only 36% of such patients, and a thymoma is present in 9%.
Diabetes mellitus and Addison disease may coexist with thyrotoxicosis and can aggravate the weight loss, fatigue, and muscle weakness seen with hyperthyroidism.
Complications
Hypercalcemia, osteoporosis, and nephrocalcinosis may occur in hyperthyroidism. Decreased libido, erectile dysfunction, diminished sperm motility, and gynecomastia may be noted in men. Other complications include cardiac arrhythmias and HF, thyroid crisis, ophthalmopathy, dermopathy, and thyrotoxic hypokalemic periodic paralysis.
Treatment
A. Treatment of Graves Disease
Table 28-5. Medications for the Treatment of Hyperthyroidism outlines the treatment options for hyperthyroidism.
Table 28-5. Medications for the treatment of hyperthyroidism.1Medication | Dose and Frequency | Indications |
|---|---|---|
Propranolol ER | Dose: 60-80 mg orally once daily, increasing every 3 days until heart rate < 90 beats per minute. Maximum dose: 320 mg daily | Symptomatic relief of tachycardia, tremor, diaphoresis, anxiety Thyrotoxic crisis Hypokalemic periodic paralysis |
Thiourea: Methimazole |
Initial dose: usually 30-60 mg orally once daily Dose may be divided and given twice daily to avoid GI upset Lower dose of 10-20 mg for very mild symptoms During pregnancy or breastfeeding, dose should not exceed 20 mg daily |
Young adults Older adult patients Mild thyrotoxicosis Small goiter Fear of isotopes Precautions during pregnancy2 |
Propylthiouracil (PTU) | Dose: 300-600 mg orally daily in four divided doses During pregnancy or breastfeeding, dose should not exceed 200 mg daily |
Precautions during pregnancy2 |
Iodinated contrast agents Iopanoic acid or ipodate sodium | Initial dose: 500 mg orally twice daily for 3 days Maintenance dose: 500 mg once daily | Effective temporary treatment of thyrotoxicosis, especially for patients who are very symptomatic Alternative treatment for patients intolerant of thioureas |
Radioactive iodine (RAI,131 I) |
| Destroys overactive thyroid tissue See text for Precautions Avoid with thyroid eye disease (Graves ophthalmopathy) |
Prednisone | Initial dose: 0.5-0.7 mg/kg orally daily After 2 weeks: begin to slowly taper and stop after about 3 months | Type 2 amiodarone-induced thyrotoxicosis |
1 See text for expanded discussion of these agents.
2 See Treatment of Hyperthyroidism During Pregnancy-Planning, Pregnancy, and Lactation in text.
1. Propranolol
Propranolol is used for symptomatic relief of tachycardia, tremor, diaphoresis, and anxiety until the hyperthyroidism is resolved. It is the initial treatment of choice for thyrotoxic crisis and effectively treats thyrotoxic hypokalemic periodic paralysis. Propranolol has no effect on thyroid hormone secretion. Treatment usually starts with propranolol ER, which is given every 12 hours for severe hyperthyroidism due to accelerated metabolism of the propranolol; it may be given once daily as hyperthyroidism improves (Table 28-5. Medications for the Treatment of Hyperthyroidism).
2. Thiourea Drugs
Methimazole or PTU is generally used for young adults or patients with mild thyrotoxicosis, small goiters, or fear of isotopes. See Treatment of Hyperthyroidism During Pregnancy-Planning, Pregnancy, and Lactation, below. Carbimazole, another thiourea that is converted to methimazole in vivo, is available outside the United States. Patients aged 65 years or older usually respond well. The drugs do not permanently damage the thyroid and are associated with a lower chance of posttreatment hypothyroidism (compared with RAI or surgery). There is a 50% chance of remission of hyperthyroidism with long-term thiourea therapy. A better likelihood of long-term remission occurs in patients with small goiters, mild hyperthyroidism, those requiring small doses of thiourea, and those with serum TSI (TSHrAb) less than 2 mU/L. Remission is marked by decreasing thiourea dosage requirement, until none is required. Patients whose TPO Ab and Tg Ab remain low after 2 years of therapy have only a 10% rate of relapse. Some clinicians advocate RAI or surgery for patients with Graves disease who continue to require thiourea therapy after 1 year. There should be no rush to discontinue thiourea therapy in favor of RAI or surgery, even after years of treatment. Thioureas may be continued long term for patients who are tolerating them well. The exception is women with thyrotoxic Graves disease who are planning pregnancy in the near future; thyroid surgery or RAI should be considered at least 4 months in advance of conception. Thiourea drugs are also useful for preparing nonpregnant hyperthyroid patients for surgery and older patients for RAI treatment.
Agranulocytosis (absolute neutrophil count below 500/mcL [0.5 × 109 /L]) or pancytopenia may occur abruptly in 0.4% of patients taking either methimazole or PTU. All patients receiving thiourea therapy must be informed of the danger of agranulocytosis or pancytopenia and the need to stop the drug and seek medical attention immediately with the onset of any infection or unusual bleeding. Nearly 85% of agranulocytosis cases occur within 90 days of commencing therapy; however, continued long-term blood test monitoring is required. Half of cases are discovered because of fever, pharyngitis, or bleeding. There is a genetic tendency to develop agranulocytosis with thiourea therapy; if a close relative has had this adverse reaction, other therapies should be considered. Agranulocytosis generally remits spontaneously with discontinuation of the thiourea. Recovery has not been improved by filgrastim (granulocyte colony-stimulating factor).
Other common side effects include pruritus, allergic dermatitis, nausea, and dyspepsia. Antihistamines may control mild pruritus without discontinuation of the drug. Since the two thiourea drugs are similar, patients who have a major allergic reaction to one should not be given the other.
The patient's thyroid status is best monitored clinically and with serum FT4 levels. The patient may become clinically hypothyroid for 2 weeks or more before TSH levels rise, the pituitary gland having been suppressed by the preceding hyperthyroidism. Rapid growth of a goiter usually occurs if prolonged hypothyroidism is allowed to develop; the goiter may sometimes become massive but usually regresses rapidly with reduction or cessation of thiourea therapy or with thyroid hormone replacement.
3. Iodinated Contrast Agents
Iopanoic acid (Telepaque) and ipodate sodium (Bilivist, Oragrafin) are iodinated contrast agents that inhibit peripheral 5'-monodeiodination of T4, thereby blocking its conversion to active T3. They provide effective temporary treatment for thyrotoxicosis of any cause and are particularly useful for patients who are symptomatically very thyrotoxic (see Thyrotoxic crisis or "thyroid storm" Psychiatric Disorders). Within 24 hours, serum T3 levels fall an average of 62%. For patients with Graves disease, methimazole is begun first to block iodine organification; the next day, ipodate sodium or iopanoic acid may be added. They offer a therapeutic option for patients with subacute thyroiditis, amiodarone-induced thyrotoxicosis, T4 overdosage, and for those intolerant to thioureas. Treatment periods of 8 months or more are possible, but efficacy tends to wane with time. In Graves disease, thyroid RAI uptake may be suppressed during treatment but typically returns to pretreatment uptake by 7 days after discontinuation of the drug, allowing 131 I treatment.
4. Lithium Carbonate
Thioureas are greatly preferred over lithium for the medical treatment of hyperthyroidism in Graves disease. However, lithium may be used effectively in cases of methimazole or PTU-induced hepatic toxicity or leukopenia. Lithium should not be used during pregnancy. Most patients require concurrent treatment with propranolol and sometimes prednisone.
5. Radioactive Iodine (Rai,131 I)
131 I therapy destroys overactive thyroid tissue (either diffuse or toxic nodular goiter). Patients who have been treated with 131 I in adulthood do not have an increased risk of subsequent thyroid cancer, leukemia, or offspring with congenital abnormalities. Conflicting evidence has shown either no increased risk or a slightly increased risk of subsequent solid tumor malignancies following 131 I treatment for hyperthyroidism.
Precautions: Because radiation is harmful to the fetus and children, RAI should not be given to pregnant or lactating women or to mothers who lack childcare. Women are advised to avoid pregnancy for at least 4 months following 131 I therapy. A pregnancy test should be obtained within 48 hours before therapy for any woman with childbearing potential. Men have been found to have abnormal spermatozoa for up to 6 months following 131 I therapy and are advised to use contraceptive methods during that time.
Patients may receive 131 I while being symptomatically treated with propranolol ER, which is then reduced in dosage as hyperthyroidism resolves. Radioiodine therapy fails to fully correct hyperthyroidism in about 20% of patients, particularly those with larger glands, very high free T4 levels, and prior thiourea therapy. However, therapy with 131 I will usually be effective if methimazole is discontinued at least 3-4 days before RAI therapy. Prior to 131 I therapy, patients are instructed against receiving intravenous iodinated contrast and should consume a low-iodine diet.
The presence of Graves ophthalmopathy is a relative contraindication to131I therapy. Following 131 I treatment for hyperthyroidism, Graves ophthalmopathy appears or worsens in 15% of patients (23% in cigarette smokers and 6% in nonsmokers) and improves in none. During treatment with methimazole, ophthalmopathy worsens in 3% and improves in 2% of patients. Therefore, patients with Graves ophthalmopathy who are to be treated with 131 I should be considered for prophylactic prednisone (20-40 mg orally daily) for 2 months following administration of 131 I; among patients receiving prednisone following 131 I treatment, preexistent ophthalmopathy improves in 67% and worsens in none.
Cigarette use increases the risk of having a flare in ophthalmopathy following 131 I treatment and also reduces the effectiveness of prednisone treatment. Patients who smoke cigarettes are strongly encouraged to quit prior to 131 I treatment. Smokers receiving 131 I should be considered for prophylactic prednisone.
FT4 levels may sometimes drop within 2 months after 131 I treatment, but then rise again to thyrotoxic levels, at which time thyroid RAI uptake is low. This phenomenon is caused by a release of stored thyroid hormone from injured thyroid cells and does not indicate a treatment failure. In fact, serum FT4 then falls abruptly to hypothyroid levels.
There is a high incidence of hypothyroidism in the months to years after 131 I. Patients with Graves disease treated with 131 I also have an increased lifetime risk of developing hyperparathyroidism, particularly when 131 I therapy was administered in childhood or adolescence. Lifelong clinical follow-up is mandatory, with measurements of serum TSH, FT4, and calcium when indicated.
6. Thyroid Surgery
Surgery may be indicated for patients with Graves disease who are intolerant to thioureas, women planning pregnancy in the near future, patients who choose not to have RAI therapy, and patients with Graves ophthalmopathy. The surgical procedure of choice is a total resection of one lobe and a subtotal resection of the other lobe, leaving about 4 g of thyroid tissue (Hartley-Dunhill operation). Subtotal thyroidectomy of both lobes ultimately results in a 9% recurrence rate of hyperthyroidism.
Patients are ordinarily rendered euthyroid preoperatively with a thiourea drug (Table 28-5. Medications for the Treatment of Hyperthyroidism). Propranolol ER is given until the heart rate is less than 90 beats per minute and continued until the serum T3 (or free T3) is normal preoperatively. If a patient undergoes surgery while thyrotoxic, larger doses of propranolol are given perioperatively to reduce the likelihood of thyroid crisis. Ipodate sodium or iopanoic acid may be used in addition to a thiourea to accelerate the decline in serum T3. The patient should be euthyroid by the time of surgery.
To reduce thyroid vascularity preoperatively, the patient may be treated for 3-10 days preoperatively with oral potassium iodide 25-50 mg (eg, ThyroShield 65 mg/mL, 0.5 mL, or SSKI 1 g/mL, 1 drop) three times daily. However, preoperative potassium iodide often increases the volume of the thyroid, so the requirement for preoperative potassium iodide for Graves disease is debatable. Preoperative iodide supplementation is not recommended prior to surgery for multinodular goiter.
The risks of subtotal or total thyroidectomy includes damage to a recurrent laryngeal nerve, with resultant vocal fold paralysis. If both recurrent laryngeal nerves are damaged, airway obstruction may develop, and the patient may require intubation and tracheostomy. Hypoparathyroidism also occurs; serum calcium levels must be checked postoperatively. Patients should be admitted for thyroidectomy surgery for at least an overnight observation period. When a competent, experienced neck surgeon performs a thyroidectomy, surgical complications are uncommon.
B. Treatment of Toxic Solitary Thyroid Nodules
Toxic solitary thyroid nodules are usually benign but may rarely be malignant. If a nonsurgical therapy is elected, the nodule should be evaluated with a fine-needle aspiration (FNA) biopsy. Medical therapy for hyperthyroidism caused by a single hyperfunctioning thyroid nodule may be treated symptomatically with propranolol ER and methimazole or PTU, as in Graves disease (Table 28-5. Medications for the Treatment of Hyperthyroidism). The dose of methimazole should be adjusted to keep the TSH slightly suppressed, so the risk of TSH-stimulated growth of the nodule is reduced. Surgical treatment is usually recommended for patients under age 40 years, for healthy older patients with toxic solitary thyroid nodules, and for nodules that are suspicious for malignancy. Patients are made euthyroid with a thiourea preoperatively and given several days of iodine, ipodate sodium, or iopanoic acid before surgery. Postoperative hypothyroidism usually resolves spontaneously, but permanent hypothyroidism occurs in about 14% of patients by 6 years after surgery. 131 I therapy may be offered to patients with a toxic solitary nodule who are over age 40 or in poor health (see Precautions for RAI use, above).
If the patient has been receiving methimazole preparatory to 131 I, the TSH should be kept slightly suppressed in order to reduce the uptake of 131 I by the normal thyroid. Nevertheless, permanent hypothyroidism occurs in about one-third of patients by 8 years after 131 I therapy. The nodule remains palpable in 50% and may grow in 10% of patients after 131 I.
C. Treatment of Toxic Nodular Goiter
Medical therapy for patients with toxic nodular goiter consists of propranolol ER (while hyperthyroid) and a thiourea, as in Graves disease (Table 28-5. Medications for the Treatment of Hyperthyroidism). Thioureas (methimazole or PTU) reverse hyperthyroidism but do not shrink the goiter. There is a 95% recurrence rate if the drug is stopped.
Surgical therapy is the definitive treatment for a large toxic nodular goiter, following therapy with a thiourea to render them euthyroid. Surgery is particularly indicated to relieve pressure symptoms or for cosmetic indications. Patients with toxic nodular goiter are not treated preoperatively with potassium iodide. Total or near-total thyroidectomy is recommended, since surgical pathology reveals unsuspected differentiated thyroid cancer in 18.3% of cases.
131 I therapy may be used to treat patients with toxic nodular goiter. See Precautions for RAI use, above. Any suspicious nodules should be evaluated beforehand for malignancy with FNA cytology. Patients are rendered euthyroid with methimazole, which is stopped 3-4 days before 131 I therapy. The approximate administered 131 I activity (dose) can be calculated for an average-size multinodular goiter, but smaller goiters require lower mcCi/g and larger goiters require higher mcCi/g dosing:
Administered Activity 131 I (*mcCi) = 150 (planned dose to thyroid in mcCi/g) × thyroid size (g) ÷ 24 h RAI uptake (decimal)
*Conversion Factor for Activity (Dose): MBq = mcCi × 0.037
The patient follows a low-iodine diet in order to enhance the thyroid gland's uptake of 131 I, which may be relatively low in this condition (compared to Graves disease). Relatively high doses of 131 I are usually required. Hypothyroidism can occur but less commonly than seen with RAI therapy for Graves disease. Recurrent thyrotoxicosis can occur, so patients must be monitored closely. Peculiarly, in about 1-5% of patients with diffusely nodular toxic goiter, the administration of 131 I therapy may induce Graves disease. Also, Graves ophthalmopathy has occurred rarely following 131I therapy for multinodular goiter.
D. Treatment of Hyperthyroidism from Thyroiditis
Patients with thyroiditis (subacute, postpartum, or silent) are treated with propranolol during the hyperthyroid phase, which usually subsides spontaneously within weeks to months. For symptomatic relief, begin propranolol ER until the heart rate is less than 90 beats per minute (Table 28-5. Medications for the Treatment of Hyperthyroidism). Ipodate sodium or iopanoic acid, 500 mg orally daily, promptly corrects elevated T3 levels and is continued for 15-60 days until the serum FT4 level normalizes. Thioureas are ineffective since thyroid hormone production is actually low in this condition. Patients are monitored carefully for the development of hypothyroidism and treated with levothyroxine as needed. With subacute thyroiditis, pain can usually be managed with NSAIDs and corticosteroids, but opioid analgesics are sometimes required.
E. Treatment of Hyperthyroidism During Pregnancy-Planning, Pregnancy, and Lactation
Due to the increased risk of congenital anomalies with every thiourea, all women who are planning to become pregnant are encouraged to consider definitive therapy with 131 I or surgery well before conception. Both men and women who are planning pregnancy should not have 131 I treatment within about 4 months of conception. See Precautions for RAI use, above. Dietary iodine must not be restricted for such women to protect the fetus from iodine deficiency.
First-trimester fetal exposure to thioureas (methimazole or PTU) increases the risk of birth defects by about 2%. The fetal anomalies associated with PTU are typically less severe than those associated with methimazole; therefore, PTU is the preferred thiourea for women actively seeking fertility and during the first trimester of pregnancy, despite the very low risk for hepatic necrosis. The most common fetal anomalies from PTU are preauricular and branchial sinus cysts and fistulas, renal cysts, hydronephrosis, intestinal bands with intestinal malrotation, and hypospadias; these anomalies are usually minor and surgically correctable. Women should be treated with PTU immediately prepregnancy and through the first trimester; during pregnancy, the dose of PTU is kept below 200 mg daily to avoid goitrous hypothyroidism in the infant. PTU can be switched to methimazole in the second trimester (see Thiourea drugs Psychiatric Disorders, above). Thiourea should be given in the smallest dose possible, permitting mild subclinical hyperthyroidism to occur, since it is usually well tolerated. About 30% of women with Graves disease experience a remission by the late second trimester.
Both PTU and methimazole cross the placenta and can induce hypothyroidism, with fetal TSH hypersecretion and goiter. Fetal ultrasound at 20-32 weeks' gestation can visualize any fetal goiter, allowing fetal thyroid dysfunction to be diagnosed and treated. Thyroid hormone administration to the mother does not prevent hypothyroidism in the fetus, since T4 and T3 do not freely cross the placenta. Fetal hypothyroidism is rare if the mother's hyperthyroidism is controlled with small daily doses of PTU (50-150 mg/day orally) or methimazole (5-15 mg/day orally). Serum total T4 levels during pregnancy should be kept at about 1.5 × the prepregnancy level. Maternal serum TSI levels over 500% at term predict an increased risk of neonatal Graves disease in the infant.
Subtotal thyroidectomy is indicated for pregnant women with Graves disease or for fertile women of reproductive age who are sexually active and decline contraceptives, under the following circumstances: (1) severe adverse reaction to thioureas; (2) high dosage requirement for thioureas (methimazole greater than or equal to 30 mg/day or PTU greater than or equal to 450 mg/day); or (3) uncontrolled hyperthyroidism due to nonadherence to thiourea therapy. Surgery is best performed during the second trimester.
Both methimazole and PTU are secreted in breast milk but not in amounts that affect the infant's thyroid hormone levels. No adverse reactions to these drugs have been reported in breast-fed infants. See Table 28-5. Medications for the Treatment of Hyperthyroidism for recommended doses. It is recommended that the medication be taken just after breastfeeding.
F. Treatment of Amiodarone-Induced Thyrotoxicosis (Ait)
Patients with either type 1 or type 2 AIT require treatment with propranolol ER for symptomatic relief and methimazole (Table 28-5. Medications for the Treatment of Hyperthyroidism). After two doses of methimazole, iopanoic acid or sodium ipodate may be added to the regimen to further block conversion of T4 to T3 until the thyrotoxicosis is resolved. If iopanoic acid or sodium ipodate is not available, potassium perchlorate may be given in doses of less than or equal to 1000 mg daily (in divided doses) for a course not to exceed 30 days to avoid the complication of aplastic anemia. Amiodarone may be withdrawn but this does not have a significant therapeutic impact for several months because of its long half-life. For patients with type 1 AIT, therapy with 131 I may be successful, but only for those with sufficient RAI uptake. Patients with type 2 AIT are usually also treated with prednisone for about 2 weeks, which is slowly tapered and withdrawn after about 3 months. Subtotal thyroidectomy should be considered for patients with AIT that is resistant to treatment.
G. Treatment of Complications
2. Cardiac Complications
HF may also occur as a result of low-output dilated cardiomyopathy. It is uncommon and may be caused by an idiosyncratic severe toxic effect of hyperthyroidism upon certain hearts. Cardiomyopathy may occur at any age and without preexisting cardiac disease. See Part 11 for treatment of HF and dilated cardiomyopathy. The patient should be rendered euthyroid. However, the HF usually persists despite correction of the hyperthyroidism.
3. Thyrotoxic Crisis or "Thyroid Storm"
ICU admission is required. A thiourea drug is given (eg, methimazole, 15-25 mg orally every 6 hours, or PTU, 150-250 mg orally every 6 hours). Ipodate sodium (500 mg/day orally) can be helpful if begun 1 hour after the first dose of thiourea. Iodide is given 1 hour later as potassium iodide (10 drops three times daily orally). Propranolol is given in a dosage of 0.5-2 mg intravenously every 4 hours or 20-120 mg orally every 6 hours. Hydrocortisone is usually given in doses of 50 mg orally every 6 hours, with rapid dosage reduction as the clinical situation improves. Plasmapheresis has been successfully used in refractory cases to directly remove thyroid hormone. Aspirin is avoided since it displaces T4 from thyroxine-binding globulin (TBG), raising FT4 serum levels. For refractory cases, emergency surgical thyroidectomy is an option.
Supportive care is usually required, including vasopressors, mechanical ventilation, dialysis, and extracorporeal membrane oxygenation (ECMO) for cardiogenic shock.
4. Hyperthyroidism from Postpartum Thyroiditis
Propranolol ER is given during the hyperthyroid phase followed by levothyroxine during the hypothyroidism phase (see Thyroiditis).
5. Graves Dermopathy
Treatment involves application of a topical corticosteroid (eg, fluocinolone) with nocturnal plastic occlusive dressings. Compression stockings may improve any associated edema.
6. Thyrotoxic Hypokalemic Periodic Paralysis
Therapy with oral propranolol, 3 mg/kg in divided doses, normalizes the serum potassium and phosphate levels and reverses the paralysis within 2-3 hours. No intravenous potassium or phosphate is usually required. Intravenous dextrose and oral carbohydrate aggravate the condition and are to be avoided. Therapy is continued with propranolol, 60-80 mg orally every 8 hours (or propranolol ER daily at equivalent daily dosage), along with a thiourea drug (eg, methimazole) to treat the hyperthyroidism.
Prognosis
Mild Graves disease may sometimes subside spontaneously. Graves disease that presents in early pregnancy has a 30% chance of spontaneous remission before the third trimester. The ocular, cardiac, and psychological complications can become serious and persistent even after treatment. Permanent hypoparathyroidism and vocal cord palsy are risks of surgical thyroidectomy. Recurrences are common following thiourea therapy but also occur after low-dose 131 I therapy or subtotal thyroidectomy. With adequate treatment and long-term follow-up, the results are usually good. However, despite treatment for hyperthyroidism, women experience an increased long-term risk of death from thyroid disease, CVD, stroke, and fracture of the femur. Posttreatment hypothyroidism is common. It may occur within a few months or up to several years after RAI therapy or subtotal thyroidectomy. Patients with thyrotoxic crisis have a high mortality rate despite treatment.
Subclinical hyperthyroidism generally subsides spontaneously. Progression to symptomatic thyrotoxicosis occurs at a rate of 1-2% per year in patients without a goiter and at a rate of 5% per year in patients with a multinodular goiter. Most patients do well without treatment and the serum TSH usually reverts to normal within 2 years. Most such patients do not have accelerated bone loss. However, if a baseline bone density shows significant osteopenia, bone densitometry may be performed periodically. In persons over age 60 years, serum TSH is suppressed (below 0.1 mIU/L) in 3% and mildly low (0.1-0.4 mIU/L) in 9%. The chance of developing atrial fibrillation is 2.8% yearly in older patients with a suppressed TSH and 1.1% yearly in those with mildly low TSH. Asymptomatic persons with very low serum TSH are monitored closely but are not treated unless atrial fibrillation or other manifestations of hyperthyroidism develop.
When to Admit
- Thyroid crisis.
- Hyperthyroidism-induced atrial fibrillation with severe tachycardia.
- Thyroidectomy.
AziziFet al. Efficacy and safety of long-term methimazole versus radioactive iodine in the treatment of toxic multinodular goiter. Endocrinol Metab (Seoul). 2022;37:861. [PMID: 36415961] BourcierSet al. Thyroid storm in the ICU: a retrospective multicenter study. Crit Care Med. 2020;48:83. [PMID: 31714398] BrancatellaAet al. Management of thyrotoxicosis induced by PD1 or PD-L1 blockade. J Endocr Soc. 2021;5:bvab093. [PMID: 34337277] CheeYJet al. SARS-CoV-2 mRNA and Graves' disease: a report of 12 cases and review of the literature. J Clin Endocrinol Metab. 2022;107:e2324. [PMID: 35235663] GriffithMLet al. Approach to the patient with thyrotoxicosis using telemedicine. J Clin Endocrinol Metab. 2020;105:dgaa373. [PMID: 32525973] GronichNet al. Cancer risk after radioactive iodine treatment for hyperthyroidism: a cohort study. Thyroid. 2020;30:243. [PMID: 31880205] KahalyGJ.Management of Graves thyroidal and extrathyroidal disease: an update. J Clin Endocrinol Metab. 2020;105:3704. [PMID: 32929476] KimBW.Does radioactive iodine therapy for hyperthyroidism cause cancer?J Clin Endocrinol Metab. 2022;107:e448. [PMID: 34555150] LeeSYet al. Hyperthyroidism: a review. JAMA. 2023;330: 1472. [PMID: 37847271] PrawSSet al. Approach to the patient with a suppressed TSH. J Clin Endocrinol Metab. 2023;108:472. [PMID: 36329632] ShalabyMet al. Predictive factors of radioiodine therapy failure in Graves' disease: a meta-analysis. Am J Surg. 2022;223:287. [PMID: 33865565] McDermottMT.Hyperthyroidism. Ann Intern Med. 2020;172:ITC49[PMID: 32252086] YlliDet al. Evaluation and treatment of amiodarone-induced thyroid disorders. J Clin Endocrinol Metab. 2021;106:226. [PMID: 33159436] YuWet al. Side effects of PTU and MMI in the treatment of hyperthyroidism: a systematic review and meta-analysis. Endocr Pract. 2020;26:207. [PMID: 31652102] |