Key Clinical Updates in Heart Failure Resynchronization therapy is indicated for patients with class II to III HF, EF of 35% or less, sinus rhythm, and left bundle branch block pattern with QRS duration of 150 msec or more. Heidenreich PA et al. J Am Coll Cardiol. [PMID: 35379503] Both empagliflozin and dapagliflozin have been shown to decrease cardiovascular mortality and HF hospitalization or worsening of HF in patients with HFpEF. Kosiborod MN et al. N Engl J Med. [PMID: 37622681] |
| ESSENTIALS OF DIAGNOSIS | ||
|
General Considerations
HF is a common syndrome that is increasing in incidence and prevalence. Approximately 6.7 million patients in the United States have HF, with 8 million or more patients projected to have HF by 2030. Each year in the United States, 1,297,000 patients are discharged from the hospital with a diagnosis of HF. It is primarily a disease of aging, with over 75% of existing and new cases occurring in individuals over 65 years of age. Seventy-five percent of HF patients have antecedent hypertension. The prevalence of HF rises from less than 1% in individuals below 60 years to nearly 10% in those over 80 years of age.
HF may be right-sided, left-sided, or both. Patients with left HF may have symptoms of low cardiac output and elevated pulmonary venous pressure; dyspnea is the predominant feature. Signs of fluid retention predominate in right HF. Most patients exhibit symptoms or signs of both right- and left-sided failure, and LV dysfunction is the primary cause of RV failure. Approximately half of patients with HF have preserved LV systolic function and usually have some degree of diastolic dysfunction. Patients with reduced or preserved systolic function may have similar symptoms and it may be difficult to distinguish clinically between the two based on signs and symptoms. In developed countries, CAD with resulting MI and loss of functioning myocardium (ischemic cardiomyopathy) is the most common cause of systolic HF. Systemic hypertension remains an important cause of HF and, even more commonly in the United States, an exacerbating factor in patients with cardiac dysfunction due to other causes, such as CAD. Several processes may present with dilated or congestive cardiomyopathy, which is characterized by LV or biventricular dilation and generalized systolic dysfunction. These are discussed elsewhere in this chapter, but the most common are alcoholic cardiomyopathy, viral myocarditis (including infections by HIV; see also the COVID-19 section in Part 34), and dilated cardiomyopathies with no obvious underlying cause (idiopathic cardiomyopathy). Rare causes of dilated cardiomyopathy include infiltrative diseases (hemochromatosis, sarcoidosis, amyloidosis, etc), other infectious agents, metabolic disorders, cardiotoxins, and medication toxicity. Valvular heart diseases-particularly degenerative aortic stenosis and chronic aortic or mitral regurgitation-are not infrequent causes of HF. Persistent tachycardia, often related to atrial arrhythmias, can cause systolic dysfunction that may be reversible with controlling the rate. Diastolic cardiac dysfunction is associated with aging and related myocardial stiffening, as well as LVH, commonly resulting from hypertension. Conditions such as hypertrophic or restrictive cardiomyopathy, diabetes, and pericardial disease can produce the same clinical picture. Atrial fibrillation with or without rapid ventricular response may contribute to impaired LV filling.
HF is often preventable by early detection of patients at risk and by early intervention. The importance of these approaches is emphasized by US guidelines that have incorporated a classification of HF that includes four stages (eTable 11-1. Stages of Hf). Stage A includes patients at risk for developing HF (such as patients with hypertension). In the majority of these patients, development of HF can be prevented with interventions such as the aggressive treatment of hypertension, modification of coronary risk factors, and reduction of excessive alcohol intake (eTable 11-1. Stages of Hf). Stage B includes patients who have structural heart disease, increased filling pressures, risk factors and elevated biomarkers, but no current or previously recognized symptoms of HF. Examples include patients with previous MI, other causes of reduced systolic function, LVH, or asymptomatic valvular disease. Both ACE inhibitors and beta-blockers prevent HF in the first two of these conditions, and more aggressive treatment of hypertension and early surgical intervention are effective in the latter two. Stages C and D include patients with clinical HF (current or previous) and the relatively small group of patients who have become refractory to the usual therapies, respectively.
eTable 11-1. Stages of HF.Stage | Description | Examples |
|---|---|---|
A | Patients at high risk for developing HF because of the presence of conditions that are strongly associated with the development of HF. Such patients have no identified structural or functional abnormalities of the pericardium, myocardium, or cardiac valves and have never shown symptoms or signs of HF. | Systemic hypertension; CAD; diabetes mellitus; history of cardiotoxic drug therapy or alcohol abuse; personal history of rheumatic fever; family history of cardiomyopathy. |
B | Patients who have developed structural heart disease that is strongly associated with the development of HF but who have never shown symptoms or signs of HF. | LVH or fibrosis; LV or hypocontractility; asymptomatic valvular heart disease; previous MI. |
C | Patients who have current or prior symptoms of HF associated with underlying structural heart disease. | Dyspnea or fatigue due to LV systolic dysfunction; asymptomatic patients who are undergoing treatment for prior symptoms of HF. |
D | Patients with advanced structural heart disease and marked symptoms of HF at rest despite maximal medical therapy and who require specialized interventions. | Patients who are frequently hospitalized for HF and cannot be safely discharged from the hospital; patients in the hospital awaiting heart transplantation; patients at home receiving continuous intravenous support for symptom release or being supported with a mechanical circulatory assist device; patients in a hospice setting for the management of HF. |
Pathophysiology
Systolic function of the heart and resulting cardiac output is governed by four major determinants: the contractile state of the myocardium, the preload of the ventricle (the end-diastolic volume and the resultant fiber length of the ventricles prior to onset of the contraction), the afterload applied to the ventricles (the impedance to LV ejection), and the heart rate.
Cardiac function may be inadequate as a result of alterations in any of these determinants. The primary derangement is often depression of myocardial contractility caused either by loss of functional muscle (due to MI, etc) or by processes diffusely affecting the myocardium. However, the heart may fail as a pump because preload is excessively elevated, such as in valvular regurgitation, or when afterload is excessive, such as in aortic stenosis or in severe hypertension. Pump function may also be inadequate when the heart rate is too slow or too rapid. Whereas the normal heart can tolerate wide variations in preload, afterload, and heart rate, the diseased heart often has limited reserve for such alterations. Finally, cardiac pump function may be supranormal but nonetheless inadequate when metabolic demands or requirements for blood flow are excessive. This situation is termed high-output HF and, though uncommon, tends to be treatable (eFigure 11-1). Causes of high output include thyrotoxicosis, severe anemia, arteriovenous shunting (including dialysis fistulas), Paget disease of bone, and thiamine deficiency (beriberi).
eFigure 11-1. High-Output Hf Secondary to Arteriovenous Fistula
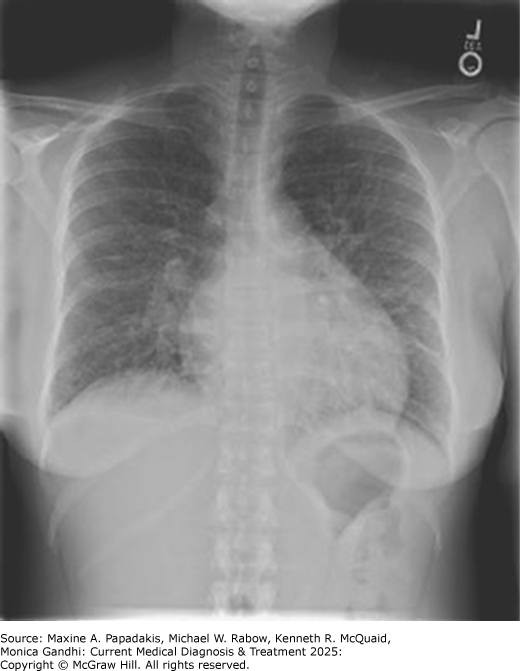
High-output HF secondary to arteriovenous fistula. PA chest film at the time of presentation demonstrating cardiomegaly, peri-bronchial cuffing, bilateral pleural effusions, and Kerley B lines. (Reproduced, with permission, from Stern AB, Klemmer PJ. High-output heart failure secondary to arteriovenous fistula. Hemodial Int. 2011;15:104.)
Manifestations of cardiac failure can also occur as a result of isolated or predominant diastolic dysfunction of the heart. In these cases, filling of the LV or RV is abnormal, either because myocardial relaxation is impaired or because the chamber is noncompliant ("stiff") due to excessive hypertrophy or changes in composition of the myocardium (VIDEO 11-1). Even though contractility may be preserved, diastolic pressures are elevated and cardiac output may be reduced, potentially causing fluid retention, dyspnea, and exercise intolerance (eFigure 11-2). Diastolic dysfunction comprises about half of all clinical HF and is especially common in older adults.
Video 11-1. Spectral pulse wave doppler examination of mitral valve inflow showing diastolic dysfunction.
(Used, with permission, from B Macrum and E Foster.)
Duration: 24 secs
Height: 360
Width: 480
Player: NULL
Player Key: NULL
Video Id: fc472f97-7669-4adb-8d42-0758705926d5
Echocardiography
eFigure 11-2. In Diastolic Dysfunction, the Diastolic Pressure-Volume Relationship is Shifted Upward and to the Left (Dashed Line), Which Leads to an Elevated Lv End-Diastolic Pressure a and Reduced Stroke Volume
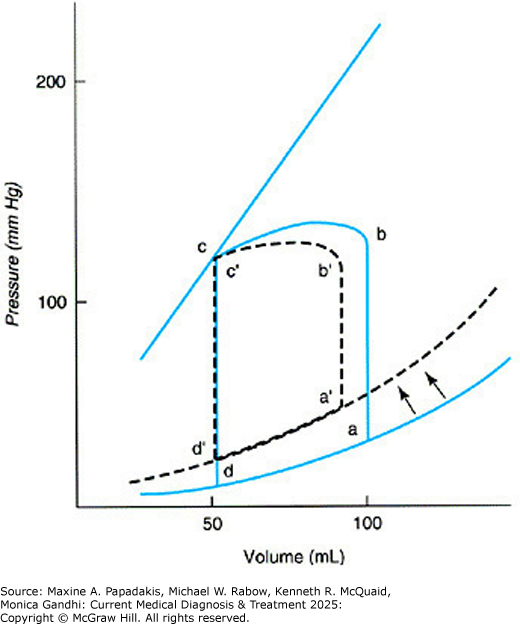
In diastolic dysfunction, the diastolic pressure-volume relationship is shifted upward and to the left (dashed line), which leads to an elevated LV end-diastolic pressure a and reduced stroke volume. (Reproduced, with permission, from Hammer GD, McPhee SJ. Pathophysiology of Disease: An Introduction to Clinical Medicine, 8th ed. McGraw-Hill, 2019.)
When the heart fails, a number of adaptations occur both in the heart and systemically. If the stroke volume of either ventricle is reduced by depressed contractility or excessive afterload, end-diastolic volume and pressure in that chamber will rise. This increases end-diastolic myocardial fiber length, resulting in a greater systolic shortening (Starling law of the heart). If the condition is chronic, ventricular dilation will occur. Although this may restore resting cardiac output, the resulting chronic elevation of diastolic pressures will be transmitted to the atria and to the pulmonary and systemic venous circulation, resulting in pulmonary or systemic edema. Reduced cardiac output, particularly if associated with reduced arterial pressure or perfusion of the kidneys, will also activate several neural and humoral systems. Increased activity of the sympathetic nervous system will stimulate myocardial contractility, heart rate, and venous tone. Although these adaptations are designed to increase cardiac output, they may themselves be deleterious. Thus, tachycardia and increased contractility may precipitate ischemia in patients with underlying CAD, and the rise in preload may worsen pulmonary congestion. Sympathetic nervous system activation also increases peripheral vascular resistance; this adaptation is designed to maintain perfusion to vital organs, but when it is excessive it may itself reduce renal and other tissue blood flow. Sympathetic activation initiates a series of myocellular events that contribute to adverse ventricular remodeling and progressive ventricular dysfunction.
Another important effect of lower cardiac output is reduction of renal blood flow and GFR, which leads to sodium and fluid retention. The renin-angiotensin-aldosterone system is also activated, leading to further increases in peripheral vascular resistance and LV afterload as well as sodium and fluid retention. HF is associated with increased circulating levels of arginine vasopressin, which also serves as a vasoconstrictor and inhibitor of water excretion. Myocardial failure is characterized by two hemodynamic derangements, and the clinical presentation is determined by their severity. The first is reduction in cardiac reserve, ie, the ability to increase cardiac output in response to increased demands imposed by exercise or even ordinary activity. The second abnormality, elevation of ventricular diastolic pressures, is primarily a result of the compensatory processes in systolic HF but is the primary derangement in diastolic HF.
Clinical Findings
A. Symptoms
The most common symptom of patients with left HF is shortness of breath, chiefly exertional dyspnea at first and then progressing to orthopnea, paroxysmal nocturnal dyspnea, and rest dyspnea. Chronic nonproductive cough, which is often worse in the recumbent position, may occur. Nocturia due to excretion of fluid retained during the day and increased renal perfusion in the recumbent position is a common nonspecific symptom of HF, as is fatigue and exercise intolerance. These symptoms correlate poorly with the degree of cardiac dysfunction. Patients with right HF have predominate signs of fluid retention, with the patient exhibiting edema, hepatic congestion and, on occasion, loss of appetite and nausea due to edema of the gut or impaired GI perfusion and ascites. Surprisingly, some individuals with severe LV dysfunction will display few signs of left HF and appear to have isolated right HF. Indeed, they may be clinically indistinguishable from patients with right HF secondary to pulmonary disease.
Patients with acute HF from MI, myocarditis, and acute valvular regurgitation due to endocarditis or other conditions usually present with pulmonary edema. Patients with episodic symptoms may be having LV dysfunction due to intermittent ischemia. Patients may also present with acute exacerbations of chronic, stable HF. Exacerbations may be caused by alterations in therapy (or patient noncompliance), excessive salt and fluid intake, arrhythmias, excessive activity, pulmonary emboli, intercurrent infection, or progression of the underlying disease.
Patients with HF are often categorized by the NYHA classification as class I (asymptomatic), class II (symptomatic with moderate activity), class III (symptomatic with mild activity), or class IV (symptomatic at rest). This classification is important since some of the treatments are indicated based on NYHA classification.
B. Signs
Many patients with HF, including some with severe symptoms, appear comfortable at rest. Others will be dyspneic during conversation or minor activity, and those with long-standing severe HF may appear cachectic or cyanotic. The vital signs may be normal, but tachycardia, hypotension, and reduced pulse pressure may be present. Patients often show signs of increased sympathetic nervous system activity, including cold extremities and diaphoresis. Important peripheral signs of HF can be detected by examination of the neck, the lungs, the abdomen, and the extremities. RA pressure may be estimated through the height of the pulsations in the jugular venous system. With the patient at 45 degrees, measure the height of the pulsation about the sternal angle, and add 5 cm to estimate the height above the left atrium, with a pressure greater than 8 cm being abnormal. In addition to the height of the venous pressure, abnormal pulsations, such as regurgitant v waves, should be sought. Examination of the carotid pulse may allow estimation of pulse pressure as well as detection of aortic stenosis. Thyroid examination may reveal occult hyperthyroidism or hypothyroidism, which are readily treatable causes of HF. Crackles at the lung bases reflect transudation of fluid into the alveoli. Pleural effusions may cause bibasilar dullness to percussion. Expiratory wheezing and rhonchi may be signs of HF. Patients with severe right HF may have hepatic enlargement-tender or nontender-due to passive congestion. Systolic pulsations may be felt in tricuspid regurgitation. Sustained moderate pressure on the liver may increase jugular venous pressure (JVP) (a positive hepatojugular reflux is an increase of greater than 1 cm, which correlates with elevated pulmonary capillary wedge pressure [PCWP]). Ascites may also be present. Peripheral pitting edema is a common sign in patients with right HF and may extend into the thighs and abdominal wall.
Cardinal cardiac examination signs are a parasternal lift, indicating pulmonary hypertension; an enlarged and sustained LV impulse, indicating LV dilation and hypertrophy; a diminished first heart sound, suggesting impaired contractility; and an S3 gallop originating in the LV and sometimes the RV. An S4 is usually present in diastolic HF. Murmurs should be sought to exclude primary valvular disease; secondary mitral regurgitation and tricuspid regurgitation murmurs are common in patients with dilated ventricles. In chronic HF, many of the expected signs of HF may be absent despite markedly abnormal cardiac function and hemodynamic measurements.
C. Laboratory Findings
A blood count may reveal anemia and a high red-cell distribution width (RDW), both of which are associated with poor prognosis in chronic HF through poorly understood mechanisms. Kidney function tests can determine whether cardiac failure is associated with impaired kidney function that may reflect poor kidney perfusion. CKD is another poor prognostic factor in HF and may limit certain treatment options. Serum electrolytes may disclose hypokalemia, which increases the risk of arrhythmias; hyperkalemia, which may limit the use of inhibitors of the renin-angiotensin system; or hyponatremia, an indicator of marked activation of the renin-angiotensin system and a poor prognostic sign. Thyroid function should be assessed to detect occult thyrotoxicosis or myxedema, and iron studies should be checked to test for hemochromatosis. In unexplained cases, appropriate biopsies may lead to a diagnosis of amyloidosis. Myocardial biopsy may exclude specific causes of dilated cardiomyopathy but rarely reveals specific reversible diagnoses.
Serum BNP is a powerful prognostic marker that adds to clinical assessment in differentiating dyspnea due to HF from noncardiac causes. Two markers-BNP and NT-proBNP-provide similar diagnostic and prognostic information. BNP is expressed primarily in the ventricles and is elevated when ventricular filling pressures are high. It is quite sensitive in patients with symptomatic HF-whether due to systolic or to diastolic dysfunction-but less specific in older patients, women, and patients with COPD. Studies have shown that BNP can help in emergency department triage in the diagnosis of acute decompensated HF, such that an NT-proBNP less than 300 pg/mL or BNP less than 100 pg/mL, combined with a normal ECG, makes HF unlikely. BNP is less sensitive and specific to diagnose HF in the chronic setting. BNP may be helpful in guiding the intensity of diuretic and a more consistent use of disease-modifying therapies, such as ACE inhibitors and beta-blockers, for the management of chronic HF. BNP, but not NT-proBNP, is increased by neprilysin inhibitors, since neprilysin degrades BNP. Thus, while NT-proBNP is still reliable, BNP should not be used to monitor degree of HF when patients are treated with sacubitril/valsartan. Worsening breathlessness or weight associated with a rising BNP (or both) might prompt increasing the dose of diuretics. However, there is no proven value in using serial natriuretic peptide measurements to guide therapy, as shown in the GUIDE-IT trial. Elevation of serum troponin, and especially of high-sensitivity troponin, is common in both chronic and acute HF, and it is associated with higher risk of adverse outcomes.
D. ECG and Chest Radiography
ECG may indicate an underlying or secondary arrhythmia, MI, or nonspecific changes that often include low voltage, intraventricular conduction defects, LVH, and nonspecific repolarization changes. CXRs provide information about the size and shape of the cardiac silhouette. Cardiomegaly is an important finding and is a poor prognostic sign. Evidence of pulmonary venous hypertension includes relative dilation of the upper lobe veins, perivascular edema (haziness of vessel outlines), interstitial edema, and alveolar fluid. In acute HF, these findings correlate moderately well with pulmonary venous pressure. However, patients with chronic HF may show relatively normal pulmonary vasculature despite markedly elevated pressures. Pleural effusions are common and tend to be bilateral or right-sided.
E. Additional Studies
The clinical diagnosis of systolic myocardial dysfunction is often inaccurate. The primary confounding conditions are diastolic dysfunction of the heart with decreased relaxation and filling of the LV (particularly in hypertension and in hypertrophic states) and pulmonary disease.
The most useful test is the echocardiogram because it can differentiate HF with and without preserved LV systolic function. The echocardiogram can define the size and function of both ventricles and of the atria. LVEF is the most commonly used measurement to define systolic function. RV function is assessed by contractility and other measures, such as tricuspid annular plane systolic excursion. Echocardiography will also allow detection of pericardial effusion, valvular abnormalities, intracardiac shunts, and segmental wall motion abnormalities suggestive of old MI as opposed to more generalized forms of dilated cardiomyopathy (VIDEO 11-2).
Video 11-2. Dilated cardiomyopathy on M-mode assessment.
(Used, with permission, from B Macrum and E Foster.)
Duration: 21 secs
Height: 360
Width: 480
Player: NULL
Player Key: NULL
Video Id: 078c252c-78bd-45e9-9756-19c25a776b25
Echocardiography
Radionuclide angiography as well as cardiac MRI also measure LVEF and permit analysis of regional wall motion. These tests are especially useful when echocardiography is technically suboptimal, such as in patients with severe pulmonary disease. MRI can assess for presence of scar tissue and of infiltrative disease. When myocardial ischemia is suspected as a cause of LV dysfunction, as it should be unless there is another clear cause, stress testing or coronary angiography should be performed.
F. Cardiac Catheterization
In most patients with HF, clinical examination and noninvasive tests can determine LV size and function and valve function to support and refine the diagnosis. Left heart catheterization may be helpful to define the presence and extent of CAD, although CT angiography may also be appropriate, especially when the likelihood of coronary disease is low. Evaluation for coronary disease is particularly important when LV dysfunction may be partially reversible by revascularization. The combination of angina or noninvasive evidence of significant myocardial ischemia with symptomatic HF is often an indication for coronary angiography if the patient is a potential candidate for revascularization. Right heart catheterization may be useful to select and monitor therapy in patients refractory to standard therapy.
Treatment: Heart Failure With Reduced Lvef
The treatment of HF is aimed at relieving symptoms, improving functional status, and preventing death and hospitalizations. The evidence of clinical benefit, including reducing death and hospitalization, as well as reducing sudden cardiac death, of most therapies is limited to patients with HF with reduced LVEF (40% or less). The SGLT-2 inhibitors, which reduce HF hospitalization for patients with preserved EF, are the one exception to this general finding. Using angiotensin receptor-neprilysin inhibitor, beta-blockers, mineralocorticoid receptor antagonists, and SGLT-2 inhibitor medication at appropriate doses for HF with reduced LVEF is estimated to reduce mortality by over 70%. Thus, this goal is as important as any in all of cardiology. It is now recognized that patients with mildly reduced EF (41-49%) may derive benefit from mineralocorticoid receptor antagonist and angiotensin receptor-neprilysin inhibitor (ARNI) (sacubitril/valsartan). Treatment of HF with preserved LVEF is aimed at improving symptoms and treating comorbidities. Achieving target (or maximally tolerated up to target) dosing to obtain the benefits of these treatments that have been shown in clinical trials is important (Table 11-1. Evidence-Based Doses of Disease-Modifying Medications in Key Randomized Trials in Hfref or after Mi (Medications Listed in Alphabetical Order Within Classes)).
Table 11-1. Evidence-based doses of disease-modifying medications in key randomized trials in HFrEF or after MI (medications listed in alphabetical order within classes).Medications | Starting Dose | Target Dose |
|---|---|---|
ACE Inhibitors | ||
Captopril | 6.25 mg three times daily | 50 mg three times daily |
Enalapril | 2.5 mg twice daily | 10-20 mg twice daily |
Lisinopril | 2.5-5.0 mg once daily | 20-35 once daily |
Ramipril | 2.5 mg once daily | 10 mg once daily |
Trandolapril | 0.5 mg once daily | 4 mg once daily |
Beta-Blockers | ||
Bisoprolol | 1.25 mg once daily | 10 mg once daily |
Carvedilol | 3.125 mg twice daily | 25 mg twice daily |
Metoprolol succinate (CR/XL) | 12.5-25 mg once daily | 200 mg once daily |
Nebivolol | 1.25 once daily | 10 mg once daily |
ARBs | ||
Candesartan | 4-8 mg once daily | 32 mg once daily |
Losartan | 50 mg once daily | 150 mg once daily |
Valsartan | 40 mg twice daily | 160 mg twice daily |
Aldosterone Antagonists | ||
Eplerenone | 25 mg once daily | 50 mg once daily |
Spironolactone | 25 mg once daily | 50 mg once daily |
ARNI | ||
Sacubitril/valsartan | 49/51 mg twice daily | 97/103 mg twice daily |
If Channel Blocker | ||
Ivabradine | 5 mg twice daily | 7.5 mg twice daily |
SGLT-2 inhibitors | ||
Dapagliflozin | 10 mg once daily | 10 mg once daily |
Empagliflozin | 10 mg once daily | 10 mg once daily |
A. Correction of Reversible Causes
The major reversible causes of HF with reduced LVEF, also called chronic systolic HF, include valvular lesions, myocardial ischemia, uncontrolled hypertension, arrhythmias (especially persistent tachycardias), alcohol- or drug-induced myocardial depression, hypothyroidism, intracardiac shunts, and high-output states. Calcium channel blockers with negative inotropy (specifically verapamil or diltiazem), antiarrhythmic medications, thiazolidinediones, and NSAIDs may be important contributors to worsening HF. Some metabolic and infiltrative cardiomyopathies may be partially reversible, or their progression may be slowed; these include hemochromatosis, sarcoidosis, and amyloidosis. Once possible reversible components are being addressed, the measures outlined below are appropriate.
B. Pharmacologic Treatment
See also the following section Acute Heart Failure & Pulmonary Edema.
1. Diuretic Therapy
Diuretics are the most effective means of providing symptomatic relief to patients with moderate to severe HF with dyspnea and fluid overload, for HF with either reduced or preserved LVEF. Few patients with symptoms or signs of fluid retention can be optimally managed without a diuretic. However, excessive diuresis can lead to electrolyte imbalance and neurohormonal activation. A combination of a diuretic and an ACE inhibitor or ARNI, along with the early addition of a beta-blocker and SGLT-2 inhibitor, should be the initial treatment in most symptomatic patients with HF and reduced LVEF.
When fluid retention is mild, thiazide diuretics or a similar type of agent (hydrochlorothiazide, 25-100 mg; metolazone, 2.5-5 mg; chlorthalidone, 25-50 mg; etc) may be sufficient. Thiazide or related diuretics often provide better control of hypertension than short-acting loop agents. The thiazides are generally ineffective when the GFR falls below 30-40 mL/min/1.73 m2 , a not infrequent occurrence in patients with severe HF. Metolazone maintains its efficacy down to a GFR of approximately 20-30 mL/min/1.73 m2 . Adverse reactions include hypokalemia and intravascular volume depletion with resulting prerenal azotemia, skin rashes, neutropenia and thrombocytopenia, hyperglycemia, hyperuricemia, and hepatic dysfunction.
Patients with more severe HF should be treated with one of the oral loop diuretics. These include furosemide (20-320 mg daily), bumetanide (1-8 mg daily), and torsemide (20-200 mg daily). These agents have a rapid onset and a relatively short duration of action. In patients with preserved kidney function, two or more daily doses are preferable to a single larger dose. In acute situations or when GI absorption is in doubt, they should be given intravenously. Torsemide may be effective when furosemide is not, related to better absorption and a longer half-life, although a large randomized trial has shown no difference in clinical outcomes between these diuretics. Larger doses (up to 500 mg of furosemide or equivalent) may be required with severe renal impairment. The major adverse reactions include intravascular volume depletion, prerenal azotemia, and hypotension. Hypokalemia, particularly with accompanying digitalis therapy, is a major problem. Less common side effects include skin rashes, GI distress, and ototoxicity (the latter more common with ethacrynic acid and possibly less common with bumetanide).
The oral potassium-sparing agents are often useful in combination with the loop diuretics and thiazides, with the first choice being the aldosterone inhibitors spironolactone (12.5-100 mg daily) or eplerenone (25-100 mg daily), which reduce mortality in addition to the diuretic effect. Aldosterone is often increased in HF. These medications spare loss of potassium, they have some diuretic effect (especially at higher doses), and they also improve clinical outcomes, including survival. Their onsets of action are slower than the other potassium-sparing agents, and spironolactone's side effects include gynecomastia and hyperkalemia. Combinations of potassium supplements or ACE inhibitors and potassium-sparing medications can increase the risk of hyperkalemia but have been used with success in patients with persistent hypokalemia.
Patients with refractory edema may respond to combinations of a loop diuretic and thiazide-like agents. Metolazone, because of its maintained activity with CKD, is the most useful agent for such a combination. Extreme caution must be observed with this approach, since massive diuresis and electrolyte imbalances often occur; 2.5 mg of metolazone orally should be added to the previous dosage of loop diuretic. In many cases this is necessary only once or twice a week, but dosages up to 10 mg daily have been used in some patients.
2. Inhibitors of the Renin-Angiotensin-Aldosterone System
Inhibition of the renin-angiotensin-aldosterone system with ACE inhibitors should be part of the initial therapy of this syndrome based on their mortality benefits.
Because ACE inhibitors may induce significant hypotension, particularly following the initial doses, they must be started with caution. Hypotension is most prominent in patients with already low BPs (systolic pressure less than 100 mm Hg), hypovolemia, prerenal azotemia (especially if it is diuretic induced), and hyponatremia (an indicator of activation of the renin-angiotensin system). These patients should generally be started at low dosages (captopril 6.25 mg orally three times daily, enalapril 2.5 mg orally daily, or the equivalent), but other patients may be started at twice these dosages. Within several days (for those with the markers of higher risk) or at most 2 weeks, patients should be questioned about symptoms of hypotension, and both kidney function and potassium levels should be monitored.
ACE inhibitors should be titrated to the dosages proved effective in clinical trials (captopril 50 mg three times daily, enalapril 10 mg twice daily, ramipril 10 mg daily, lisinopril 20 mg daily, or the equivalent) over a period of 1-3 months. Most patients will tolerate these doses. Asymptomatic hypotension is not a contraindication to up-titrating or continuing ACE inhibitors. Some patients exhibit increases in serum creatinine or potassium, but they do not require discontinuation if the levels stabilize-even at values as high as 3 mg/dL and 5.5 mEq/L, respectively. Kidney dysfunction is more frequent in patients with diabetes, older patients, and those with low systolic pressures, and these groups should be monitored more closely. The most common side effects of ACE inhibitors in HF patients are dizziness (often not related to the level of BP) and cough, though the latter is often due as much to HF or intercurrent pulmonary conditions as to the ACE inhibitor. ACE inhibitor-induced cough is more common in women than in men.
However, these agents do not share the effects of ACE inhibitors on other potentially important pathways that produce increases in bradykinin, prostaglandins, and nitric oxide in the heart, blood vessels, and other tissues. The Valsartan in Heart Failure Trial (Val-HeFT) examined the efficacy of adding valsartan (titrated to a dose of 160 mg orally twice a day) to ACE inhibitor therapy. While the addition of valsartan did not reduce mortality, the composite of death or hospitalization for HF was significantly reduced. The CHARM trial randomized 7601 patients with chronic HF with or without LV systolic dysfunction and with or without background ACE inhibitor therapy to candesartan (titrated to 32 mg orally daily) or placebo. Among patients with an LVEF of less than 40%, there was an 18% reduction in cardiovascular death or HF hospitalization and a statistically significant 12% reduction in all-cause mortality. The benefits were similar among patients taking ACE inhibitors, including among patients taking full-dose ACE inhibitors. ARBs, specifically candesartan or valsartan, provide important benefits as an alternative to ACE inhibitors in chronic HF with reduced LVEF. (A large trial of patients with chronic HF and preserved LVEF found no benefit from the ARB irbesartan.) While they have the same level of recommendation in the guidelines, generally ACE inhibitors are preferred over ARBs for patients who tolerate them, although starting an ARB will avoid the need for a washout period when transitioning to sacubitril/valsartan.
While the TOPCAT trial failed to show that spironolactone improved cardiovascular mortality and morbidity in a population of patients with HF and preserved LVEF (45% or more), there appeared to be a reduction in HF hospitalization, but at the cost of increased hyperkalemia and kidney dysfunction.
This evidence led to a class I recommendation by the ACC/AHA and the European Society of Cardiology (ESC) guidelines for the use of sacubitril/valsartan as a replacement for ACE inhibitors for patients with HF with reduced EF who remain symptomatic on an ACE inhibitor, beta-blocker, and mineralocorticoid inhibitor. Patients with baseline systolic BP less than 100 mm Hg were not included in the PARADIGM trial, and symptomatic hypotension is more common with sacubitril/valsartan than ACE inhibitor. Sacubitril/valsartan can be safely started in the hospital for patients admitted with decompensated failure, once they are stable with systolic BP of at least 100 mm Hg and there has been a 36-hour washout period since the last dose of ACE inhibitor.
While there was some evidence of benefit, sacubitril/valsartan did not result in significant improvement in the primary outcome of total HF hospitalizations and cardiovascular death in the PARAGON-HF trial studying a population of patients with HF and preserved LVEF (45% or greater). However, the FDA has approved sacubitril/valsartan in this population, particularly for patients with EF "below normal," that is for EF less than 50% including patients with mildly reduced EF (41-49%).
3. Beta-Blockers
Beta-blockers are part of the foundation of care of chronic HF based on their life-saving benefits. The mechanism of this benefit remains unclear, but it is likely that chronic elevations of catecholamines and sympathetic nervous system activity cause progressive myocardial damage, leading to worsening LV function and dilation. The primary evidence for this hypothesis is that over a period of 3-6 months, beta-blockers produce consistent substantial rises in EF (averaging 10% absolute increase) and reductions in LV size and mass.
Clinical trial results have been reported in nearly 14,000 patients (ranging from asymptomatic post-MI LV dysfunction to severe HF with LVEFs less than 35-40%) receiving ACE inhibitors and diuretics randomized to beta-blockers or placebo. Three medications have strong evidence of reducing mortality: carvedilol (a nonselective beta-1- and beta-2-receptor blocker), the beta-1-selective extended-release agent metoprolol succinate (but not short-acting metoprolol tartrate), and bisoprolol (beta-1-selective agent).
Carvedilol, a nonselective beta-1- and beta-2-receptor blocker with additional weak alpha-blocking activity, was the first beta-blocker approved for HF in the United States after showing a reduction in death and hospitalizations in four smaller studies with a total of nearly 1100 patients. Subsequently, trials with two beta-1-selective agents, bisoprolol (CIBIS II, with 2647 patients) and sustained-release metoprolol succinate (MERIT, with nearly 4000 patients), showed 35% reductions in mortality as well as fewer hospitalizations. A trial using carvedilol in 2200 patients with severe (NYHA class III/IV) HF was terminated ahead of schedule because of a 35% reduction in mortality. In these trials, there were reductions in sudden deaths and deaths from worsening HF, and benefits were seen in patients with underlying coronary disease and those with primary cardiomyopathies. In all these studies, the beta-blockers were generally well tolerated, with similar numbers of withdrawals in the active and placebo groups. There is a strong recommendation that stable patients (defined as having no recent deterioration or evidence of volume overload) with mild, moderate, and even severe HF should be treated with a beta-blocker unless there is a noncardiac contraindication. In the COPERNICUS trial, carvedilol was both well tolerated and highly effective in reducing both mortality and HF hospitalizations in a group of patients with severe (NYHA class III or IV) symptoms, but care was taken to ensure that they were free of fluid retention at the time of initiation. In this study, one death was prevented for every 13 patients treated for 1 year-as dramatic an effect as has been seen with a pharmacologic therapy in the history of cardiovascular medicine. One trial comparing carvedilol and (short-acting) metoprolol tartrate (COMET) found significant reductions in all-cause mortality and cardiovascular mortality with carvedilol. Thus, patients with chronic HF should be treated with extended-release metoprolol succinate, bisoprolol, or carvedilol but not short-acting metoprolol tartrate.
Because even apparently stable patients may deteriorate when beta-blockers are initiated, initiation must be done gradually and with great care. Carvedilol is initiated at a dosage of 3.125 mg orally twice daily and may be increased to 6.25, 12.5, and 25 mg twice daily at intervals of approximately 2 weeks. The protocols for sustained-release metoprolol use were started at 12.5 or 25 mg orally daily and doubled at intervals of 2 weeks to a target dose of 200 mg daily (using the Toprol XL sustained-release preparation). Bisoprolol was administered at a dosage of 1.25, 2.5, 3.75, 5, 7.5, and 10 mg orally daily, with increments at 1- to 4-week intervals. More gradual up-titration is often more convenient and may be better tolerated. The SENIORS trial of 2135 patients found that nebivolol was effective in older adult patients (70 years and older) with chronic HF, although the evidence of degree of benefit was not as strong as with the three proven beta-blockers carvedilol, metoprolol succinate, or bisoprolol.
Patients should be instructed to monitor their weight at home as an indicator of fluid retention and to report any increase or change in symptoms immediately. Before each dose increase, patients should be seen and examined to ensure that there has not been fluid retention or worsening of symptoms. If HF worsens, this can usually be managed by increasing diuretic doses and delaying further increases in beta-blocker doses, though downward adjustments or discontinuation is sometimes required. Carvedilol, because of its beta-blocking activity, may cause dizziness or hypotension. This can usually be managed by reducing the doses of other vasodilators and by slowing the pace of dose increases.
4. Sglt-2 Inhibitors
Four large clinical trials, two with patients with HF and reduced LVEF and two with preserved LVEF, have shown that dapagliflozin and empagliflozin, inhibitors of SGLT-2, substantially reduce the risk of cardiovascular death and hospitalization for HF for patients with reduced or preserved LVEF, as well as with or without diabetes. Each medication is used in a single dose, 10 mg a day, and each results in rapid benefits (within 2 weeks) and is well tolerated with respect to blood pressure and renal function. SGLT-2 inhibitors also reduced kidney disease progression, and patients with eGFR of 20 mL/min/1.73 m2 have been included in these trials.
5. Digitalis Glycosides
The efficacy of digitalis glycosides in reducing the symptoms of HF has been established in at least four multicenter trials that have demonstrated that digoxin withdrawal is associated with worsening symptoms and signs of HF, more frequent hospitalizations for decompensation, and reduced exercise tolerance. This was also seen in the 6800-patient Digitalis Investigators Group (DIG) trial, though that study found no benefit (or harm) with regard to survival. A reduction in deaths due to progressive HF was balanced by an increase in deaths due to ischemic and arrhythmic events. Digoxin should be considered for patients who remain symptomatic when taking diuretics and ACE inhibitors as well as for patients with HF who are in atrial fibrillation and require rate control. However, there is uncertainty about the safety of digoxin in this population with atrial fibrillation, especially with higher digoxin concentrations.
Digoxin has a half-life of 24-36 hours and is eliminated almost entirely by the kidneys. The oral maintenance dose may range from 0.125 mg three times weekly to 0.5 mg daily. It is lower in patients with kidney dysfunction, in older patients, and in those with smaller lean body mass. Although an oral loading dose of 0.75-1.25 mg (depending primarily on lean body size) over 24-48 hours may be given if an early effect is desired, in most patients with chronic HF it is sufficient to begin with the expected maintenance dose (usually 0.125-0.25 mg daily). Amiodarone, quinidine, propafenone, and verapamil are among the medications that may increase digoxin levels up to 100%. It is prudent to measure a blood level after 7-14 days (and at least 6 hours after the last dose was administered). Optimum serum digoxin levels are 0.7-1.2 ng/mL. Digoxin may induce ventricular arrhythmias, especially when hypokalemia or myocardial ischemia is present (eFigure 11-3). Once an appropriate maintenance dose is established, subsequent levels are usually not indicated unless there is a change in kidney function or medications that affects digoxin levels or a significant deterioration in cardiac status that may be associated with reduced clearance. Digoxin toxicity is discussed in Part 40.
eFigure 11-3. Normal Sinus Rhythm with First-Degree Atrioventricular (AV) Block
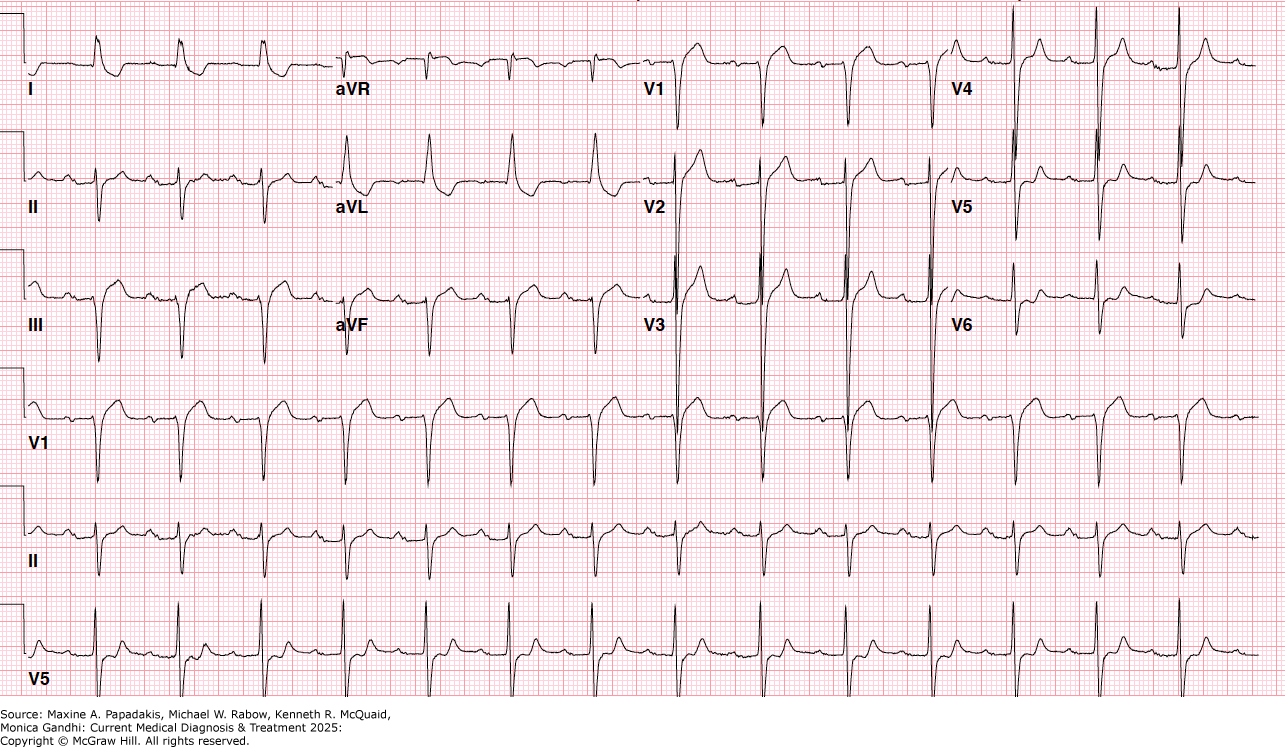
Normal sinus rhythm with first-degree atrioventricular (AV) block. There are downsloping "scooped" ST segments in I and aVL as well as biphasic T waves V5-V6 that are characteristic of digitalis toxicity. (Reproduced with permission from Jose Sanchez, MD.)
Digoxin toxicity has become less frequent as there has been a better appreciation of its pharmacology, but the therapeutic-to-toxic ratio is quite narrow. Symptoms of digitalis toxicity include anorexia, nausea, headache, blurring or yellowing of vision, and disorientation. Cardiac toxicity may take the form of AV conduction or sinus node depression; junctional, atrial, or ventricular premature beats or tachycardias; or ventricular fibrillation. Potassium administration (following serum potassium measurement, since severe toxicity may be associated with hyperkalemia) is usually indicated for the tachyarrhythmias even when levels are in the normal range, but may worsen conduction disturbances. Lidocaine or phenytoin may be useful for ventricular arrhythmias, as is overdrive pacing, but quinidine, amiodarone, and propafenone should be avoided because they will increase digoxin levels. Electrical cardioversion should be avoided if possible, particularly if there is any suspicion of digoxin toxicity, as it may cause intractable ventricular fibrillation or cardiac standstill. Pacing is indicated for third-degree AV block (complete heart block) and symptomatic or severe block (heart rate less than 40 beats/min) if they persist after treatment with atropine. Digoxin immune fab (ovine) is available for life-threatening toxicity or large overdoses, but it should be remembered that its half-life is shorter than that of digoxin and so repeat administration may be required.
6. Nitrates and Hydralazine
The combination of hydralazine and isosorbide dinitrate has been shown to improve outcomes in self-identified Black persons. ARBs or ARNIs have largely supplanted the use of the hydralazine-isosorbide dinitrate combination in patients with intolerance to ACE inhibitors. The African-American Heart Failure Trial (A-HeFT) was a large RCT investigating hydralazine (75 mg) and isosorbide dinitrate (40 mg) three times a day versus placebo on top of standard therapy in 1050 self-identified Black participants with NYHA class III or IV chronic HF. The primary endpoint was a composite score based on death from any cause, a first hospitalization for HF, and change in the quality of life. The trial was stopped early because of a significant 43% reduction in all-cause mortality with hydralazine and nitrates. The 2016 European guidelines give hydralazine and isosorbide dinitrate a class Iia recommendation for Black patients who remain symptomatic while taking an ACE inhibitor, beta-blocker, and aldosterone antagonist. They give a modest class Iib recommendation for patients with reduced LVEF who are unable to tolerate ACE inhibitor and ARB therapy or who have persistent symptoms despite treatment with a beta-blocker, ACE inhibitor, and aldosterone antagonist.
See section Acute MI with ST-Segment Elevation for a discussion on the intravenous vasodilating medications and their dosages.
Isosorbide dinitrate, 20-40 mg orally three times daily, and nitroglycerin ointment, 2%, 15-16 mg (1.4 inches; 1 inch=15 mg) every 6-8 hours, appear to be equally effective, although the ointment is generally reserved for inpatient use only. The nitrates are moderately effective in relieving shortness of breath, especially in patients with mild to moderate symptoms, but less successful-probably because they have little effect on cardiac output-in advanced HF. Nitrate therapy is generally well tolerated, but headaches and hypotension may limit the dose of all agents. The development of tolerance to long-term nitrate therapy occurs. This is minimized by intermittent therapy, especially if a daily 8- to 12-hour nitrate-free interval is used, but probably develops to some extent in most patients receiving these agents. Transdermal nitroglycerin patches have no sustained effect in patients with HF and should not be used for this indication.
Hydralazine therapy is frequently limited by side effects. Approximately 30% of patients are unable to tolerate the relatively high doses required to produce hemodynamic improvement in HF (200-400 mg daily in divided doses). The major side effect is GI distress, but headaches, tachycardia, and hypotension are relatively common. ARBs have largely supplanted the use of the hydralazine-isosorbide dinitrate combination in ACE-intolerant patients.
7. Ivabradine
Ivabradine inhibits the If channel in the sinus node and has the specific effect of slowing sinus rate. The SHIFT trial enrolled 6588 patients with symptomatic HF, LVEF of 35% or less, and sinus rhythm with rate of 70 beats/min or more. Most patients were receiving an ACE inhibitor, a beta-blocker, and an aldosterone antagonist, although a minority were on full-dose beta-blocker. Cardiovascular death and hospitalization for HF were reduced by 18%, with an absolute reduction of 4.2% over 23 months, mainly driven by less HF hospitalization. Ivabradine is approved by the FDA for use in stable patients with HF and heart rate of 70 beats/min who are taking the maximally tolerated dose of beta-blockers or in patients in whom beta-blockers are contraindicated. It is approved by the European Medicines Agency for use in patients with a heart rate of 75 beats/min or more. Both the US and the European guidelines give it a class IIa recommendation for patients in sinus rhythm with a heart rate of 70 beats/min or more with an EF of 35% or less, and persisting symptoms despite treatment with an evidence-based dose of beta-blocker (or a maximum tolerated dose below that), ACE inhibitor (or ARB), and an aldosterone antagonist (or ARB). In a trial of patients with chronic angina, ivabradine did not reduce cardiovascular events, and there may have been more events with ivabradine (than placebo) in patients with symptomatic angina.
8. Vericiguat (a Soluble Guanylate Cyclase Stimulator)
Vericiguat is FDA-approved to reduce the risk of cardiovascular death and HF hospitalization following a hospitalization for HF in patients with chronic HF and LVEF less than 45%. The VICTORIA trial showed a modest but significant reduction in cardiovascular death and HF hospitalization with vericiguat, added to other effective therapies, in this population with high-risk population.
9. Combination of Medical Therapies
Optimal management of chronic HF involves using combinations of proven life-saving therapies. Patients with HF and reduced LVEF should be treated with all four life-saving medications: beta-blockers, mineralocorticoid (aldosterone) receptor antagonists, sacubitril/valsartan, and SGLT-2 inhibitors. This combination, titrated to full tolerated doses, with careful monitoring of kidney function and potassium, will provide the greatest pharmacologic benefit to the majority of patients with HF with reduced LVEF. Achieving this goal has been shown to be more effective using a systematic approach with care pathways and frequent clinic visits. There are advantages to starting all of these medications before hospital discharge for patients hospitalized with HF, when possible.
10. Treatments that May Cause Harm in Hf with Reduced Lvef
Several therapies should be avoided, when possible, in patients with systolic HF. These include thiazolidinediones (glitazones) that cause worsening HF, most calcium channel blockers (with the exception of amlodipine and felodipine), NSAIDs, and cyclooxygenase-2 inhibitors that cause sodium and water retention and renal impairment, and the combination of an ACE inhibitor, ARB, and aldosterone blocker that increases the risk of hyperkalemia.
11. Anticoagulation
Patients with LV failure and reduced EF are at somewhat increased risk for developing intracardiac thrombi and systemic arterial emboli. However, this risk appears to be primarily in patients who are in atrial fibrillation, who have had thromboemboli, or who have LV thrombus. DOACs appear to be as effective as warfarin for patients with LV thrombus. Other patients with HF have embolic rates of approximately two per 100 patient-years of follow-up, which approximates the rate of major bleeding, and routine anticoagulation is not warranted except in patients with prior embolic events or mobile LV thrombi. A clinical trial of low-dose rivaroxaban failed to show substantial benefit in patients with HF with reduced LVEF. For patients with LV thrombi, there has been more experience with warfarin than DOACs, although the outcomes appear to be similar with DOACs in this population.
12. Antiarrhythmic Therapy
Patients with moderate to severe HF have a high incidence of both symptomatic and asymptomatic arrhythmias. Although less than 10% of patients have syncope or presyncope resulting from ventricular tachycardia, ambulatory monitoring reveals that up to 70% of patients have asymptomatic episodes of nonsustained ventricular tachycardia. These arrhythmias indicate a poor prognosis independent of the severity of LV dysfunction, but many of the deaths are probably not arrhythmia related. Beta-blockers, because of their marked favorable effect on prognosis in general and on the incidence of sudden death specifically, should be initiated in these as well as all other patients with HF (see Beta-Blockers Coronary Artery Disease, Valvular Disease, & Other Key Topics in Cardiology above). Other evidence-based therapies for HF, including ACE inhibitors, ARBs, mineralocorticoid receptor antagonists, ARNIs, and SGLT-2 inhibitors all appear to reduce sudden cardiac death. Empiric antiarrhythmic therapy with amiodarone did not improve outcome in the SCD-HeFT trial, and most other agents are contraindicated because of their proarrhythmic effects in this population and their adverse effect on cardiac function. For patients with systolic HF and atrial fibrillation, a rhythm control strategy has not been shown to improve outcome compared to a rate control strategy and thus should be reserved for patients with a reversible cause of atrial fibrillation or refractory symptoms. Then, amiodarone is the medication of choice.
C. Nonpharmacologic Treatment
1. Implantable Cardioverter Defibrillators (Icds)
Indications for ICDs include not only patients with symptomatic or asymptomatic arrhythmias but also patients with chronic HF and LV systolic dysfunction who are receiving contemporary HF treatments, including beta-blockers. In the second Multicenter Automatic Defibrillator Implantation Trial (MADIT II), 1232 patients with prior MI and an EF less than 30% were randomized to an ICD or a control group. Mortality was 31% lower in the ICD group, which translated into 9 lives saved for each 100 patients who received a device and were monitored for 3 years. The Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) reinforced and extended these results, showing a 23% relative (7.2% absolute) reduction in mortality over 5 years with a simple single-lead ICD in a population of patients with symptomatic chronic HF and an EF of 35% or less. Patients with class II symptoms appeared to have even larger benefits than patients with class III symptoms. The Centers for Medicare and Medicaid Services provides reimbursement coverage to include patients with chronic HF and ischemic or nonischemic cardiomyopathy with an EF of 35% or less. Despite a trial published in 2016 that found no survival benefit of ICDs in a population of patients with nonischemic cardiomyopathy, over half of them had cardiac resynchronization therapy, and an overview has suggested that benefit is similar for patients with ischemic or nonischemic cardiomyopathy. US guidelines continue to recommend, as class I, ICDs in for nonischemic cardiomyopathy.
2. Biventricular Pacing (Resynchronization)
Many patients with HF due to systolic dysfunction have abnormal intraventricular conduction that results in dyssynchronous and hence inefficient contractions. Several studies have evaluated the efficacy of "multisite" pacing, using leads that stimulate the RV from the apex and the LV from the lateral wall via the coronary sinus. Patients with wide QRS complexes (generally 150 msec or more), reduced EFs, and moderate to severe symptoms have been evaluated. Results from trials with up to 2 years of follow-up have shown an increase in EF, improvement in symptoms and exercise tolerance, and reduction in death and hospitalization. The COMPANION trial included 1520 patients with NYHA class III or IV HF, EF of 35% or less, and QRS duration of 120 msec or more. In addition to optimal medical therapy, resynchronization therapy with biventricular pacing with or without implantable defibrillator capability reduced death and hospitalization from any cause by about 20%. The CARE-HF trial randomized 813 similar patients, who also required mechanical evidence of dyssynchrony if QRS duration was 120-149 msec, to resynchronization therapy. Over a mean follow-up of 29 months, death or hospitalization for cardiac cause was reduced by 37% and mortality was reduced by 36%. The MADIT-CRT trial showed reduced HF events (but not mortality) with cardiac resynchronization therapy among patients with mild HF symptoms, LVEF of 30% or less, and QRS of 130 msec or more. The RAFT trial, however, showed a survival benefit to patients with mild to moderate HF symptoms. Patients with NYHA class II or III HF, LVEF of 30% or less, and a QRS duration of 120 msec or more were randomized to an ICD alone or an ICD plus cardiac resynchronization therapy. The primary outcome (death from any cause or hospitalization for HF) was reduced by 25%, as was overall mortality. The best responders to cardiac resynchronization therapy are patients with wider QRS, left bundle branch block, and nonischemic cardiomyopathy, and the lowest responders are those with narrow QRS and non-left bundle branch block pattern. Thus, as recommended in the 2022 AHA/ACC/HFSA guidelines, resynchronization therapy is indicated for patients with class II to III HF, EF of 35% or less, sinus rhythm, and left bundle branch block pattern with QRS duration of 150 msec or more.
3. Case Management, Diet, and Exercise Training
Thirty to 50 percent of HF patients who are hospitalized will be readmitted within 3-6 months. Strategies to prevent clinical deterioration, such as case management, home monitoring of weight and clinical status, and patient adjustment of diuretics, can prevent rehospitalizations and should be part of the treatment regimen of advanced HF. Involvement of a multidisciplinary team (rather than a single physician) and in-person (rather than just telephonic) communication appear to be important features of successful programs. Initiating life-saving medications during hospitalization for HF with rapid titration after discharge may improve outcomes.
Patients should routinely practice moderate salt restriction (2-2.5 g sodium or 5-6 g salt per day). More severe sodium restriction is usually difficult to achieve and unnecessary because of the availability of potent diuretic agents.
Exercise training improves activity tolerance in significant part by reversing the peripheral abnormalities associated with HF and deconditioning. In severe HF, restriction of activity may facilitate temporary recompensation. A large trial (HF ACTION, 2331 patients) showed no significant benefit (nor harm) from a structured exercise training program on death or hospitalization, although functional status and symptoms were improved. Thus, in stable patients, a prudent increase in activity or a regular exercise regimen can be encouraged. Indeed, a gradual exercise program is associated with diminished symptoms and substantial increases in exercise capacity.
Some integrative medicine approaches may be relevant in the care of patients with HF. There are clinical and epidemiologic studies that have indicated that fish oil is beneficial for patients with HF. The GISSI-HF study was a large (n=6975) double-blind, randomized trial investigating the effect of low-dose fish oil supplementation on patients with HF. Results showed that the treatment was well tolerated and associated with a significant reduction of all-cause mortality and hospitalization due to cardiovascular causes. Four other randomized trials have reported that fish oil supplementation in patients with HF increases LV end-diastolic volume, lowers serum BNP, reduces TNF, helps prevent cardiac cachexia, and improves endothelium-dependent vasodilation. A British study concluded that omega-3 fatty acid supplementation is cost-effective in patients with HF. AHA guidelines indicate that treatment with omega-3 fatty acids is reasonable for the secondary prevention of outcomes in patients with HF.
The Q-SYMBIO trial provides the strongest evidence to support the use of CoQ10 for HF. This prospective, multicenter randomized trial evaluated aCoQ10 supplementation (100 mg three times daily) over 2 years in 420 patients with moderate to severe HF. The study found CoQ10 treatment was safe, improved symptoms, and reduced major adverse cardiac events. A 2017 meta-analysis of 13 randomized trials found evidence that tai chi significantly improved 6-minute walking distance, LVEF, serum BNP, and quality of life.
4. Coronary Revascularization
Since underlying CAD is the cause of HF in the majority of patients, coronary revascularization has been thought to be able to both improve symptoms and prevent progression. While the STITCH trial failed to show an overall survival benefit from CABG among patients with multivessel coronary disease who were candidates for CABG, but who also had HF and an LVEF of 35% or less, at 5 years, there was benefit at 10 years of follow-up. Thus, revascularization does appear warranted for some patients with HF, including those with more severe angina or left main coronary disease (excluded from the STITCH trial).
5. Cardiac Transplantation
Because of the poor prognosis of patients with advanced HF, cardiac transplantation is widely used. Many centers have 1-year survival rates exceeding 80-90%, and 5-year survival rates above 70%. Infections, hypertension and kidney dysfunction caused by cyclosporine, rapidly progressive coronary atherosclerosis, and immunosuppressant-related cancers have been the major complications. The high cost and limited number of donor organs require careful patient selection early in the course.
6. Other Surgical Treatment Options
Externally powered and implantable ventricular assist devices can be used in patients who require ventricular support either to allow the heart to recover or as a bridge to transplantation. The latest generation devices are small enough to allow patients unrestricted mobility and even discharge from the hospital. Continuous flow devices appear to be more effective than pulsatile flow devices. However, complications are frequent, including bleeding, thromboembolism, and infection, and the cost is very high, exceeding $200,000 in the initial 1-3 months.
Although 1-year survival was improved in the REMATCH randomized trial, all 129 patients died by 26 months. Newer-generation continuous flow pump ventricular assist devices have been shown to result in better survival than the first-generation pulsatile flow device used in REMATCH.
Cardiomyoplasty and ventricular reduction surgery in end-stage patients have not been shown to improve prognosis or symptoms in controlled studies, and for these reasons they are not used.
7. Palliative Care
Despite the technologic advances of recent years, it should be remembered that many patients with chronic HF are older adults and have multiple comorbidities. Many of them will not experience meaningful improvements in survival with aggressive therapy. The goal of management for these patients and all those with serious illness should include symptomatic improvement and palliative care as they approach the end of life (see Part 5).
Treatment: Heart Failure With Preserved Lvef
Although half of all HF occurs among patients with normal LVEF, often with diastolic dysfunction, the only therapy shown to reduce cardiovascular death or HF hospitalization in this population is SGLT-2 inhibitors, specifically dapagliflozin or empagliflozin. The mainstays of treating HF with preserved EF are SGLT-2 inhibitors and diuretic therapy for fluid overload. Treating comorbidities like hypertension, diabetes, obesity, and arrhythmias (such as atrial fibrillation and high burden PVCs) is also important.
A. Correction of Reversible Causes
Hypertension, pericardial disease, and atrial tachycardias are potentially reversible factors that can contribute to HF with preserved LVEF. Since tachycardia is associated with shorter overall diastolic filling time, controlling accelerated heart rate may be important. With effective treatment available for familial and wild type transthyretin amyloid cardiomyopathy, this diagnosis should be considered for patients with unexplained HF with preserved EF. Treating obesity, in particular with GLP-1 receptor agonists, has shown promise to improve HF symptoms and exercise function.
B. Pharmacologic Treatment
1. Diuretic Therapy
Diuretics are important to control symptoms of fluid overload in patients with HF with preserved LVEF, similar to symptoms from systolic HF.
2. Sglt-2 Inhibitors
Both empagliflozin and dapagliflozin have been shown to decrease cardiovascular mortality and heart failure hospitalization or worsening of HF of patients with HFpEF. They cause mild diuresis and mild reduction in blood pressure, thus care is needed during initiation for patients who may be dehydrated or have low blood pressure.
3. Inhibitors of the Renin-Angiotensin-Aldosterone System
ACE inhibitors and ARBs have not been shown to improve outcome in patients with HF and preserved LVEF, despite being good therapies for the comorbidity of hypertension. Sacubitril/valsartan does not substantially improve outcome in patients with HF and preserved LVEF, although does appear to improve outcome for patients with mildly reduced LVEF (41-50%). Spironolactone has not been shown to improve outcome in a large trial of patients with HF and preserved LVEF, but there may have been some benefit in patients enrolled in the Americas who had more clearly defined HF. Spironolactone should remain a therapeutic option, especially for patients who also have hypertension. The 2017 US HF guideline gives spironolactone a class IIb recommendation for HF with preserved EF, stating that "In appropriately selected patients with HFpEF (with LVEF greater than or equal to 45%, elevated BNP levels or HF admission within 1 year, eGFR greater than 30 mL/min/1.73 m2 , creatinine less than 2.5 mg/dL, potassium less than 5.0 mEq/L), aldosterone receptor antagonists might be considered to decrease hospitalizations."
C. Nonpharmacologic Treatment
Unlike in patients with HF and reduced LVEF, ICD and resynchronization device treatments do not have a role in patients with preserved LVEF. Revascularization for patients with HF and preserved LVEF should be guided by the same considerations as for patients with HF with reduced LVEF.
Prognosis
Once manifested, HF with reduced LVEF carries a poor prognosis. Even with appropriate treatment, the 5-year mortality is approximately 50%. Mortality rates vary from less than 5% per year in those with no or few symptoms to greater than 30% per year in those with severe and refractory symptoms. These figures emphasize the critical importance of early detection and intervention. Higher mortality is related to older age, lower LVEF, more severe symptoms, CKD, and diabetes. The prognosis of HF has improved in the past two decades, probably at least in part because of the more widespread use of ACE inhibitors and beta-blockers, which markedly improve survival in those with HF with reduced LVEF.
When to Refer
Patients with new symptoms of HF not explained by an obvious cause should be referred to a cardiologist. Patients with continued symptoms of HF and reduced LVEF (35% or less) should be referred to a cardiologist for consideration of placement of an ICD or cardiac resynchronization therapy (if QRS duration is 120 msec or more, especially with left bundle branch block pattern).
When to Admit
- Patients with unexplained new or worsened symptoms or positive cardiac biomarkers concerning for acute myocardial necrosis.
- Patients with hypoxia, gross fluid overload, or pulmonary edema not readily resolved in an outpatient setting.
Al-KhatibSMet al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018;138:e210. [PMID: 29084733] AnkerSDet al; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451. [PMID: 34449189] ArmstrongPWet al; VICTORIA Study Group. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382:1883. [PMID: 32222134] BozkurtBet al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail. 2021;27:387. [PMID: 33663906] ChandraAet al. Effects of sacubitril/valsartan on physical and social activity limitations in patients with heart failure: a secondary analysis of the PARADIGM-HF trial. JAMA Cardiol. 2018;3:498. [PMID: 29617523] HeidenreichPAet al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79:1757. [PMID: 35379504] KosiborodMNet al; STEP-HFpEF Trial Committees and Investigators. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med. 2023;389:1069. [PMID: 37622681] LopesRDet al. Digoxin and mortality in patients with atrial fibrillation. J Am Coll Cardiol. 2018;71:1063. [PMID: 29519345] McDonaghTAet al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022;24:4. [PMID: 35083827] McMurrayJJVet al; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995. [PMID: 31535829] PackerMet al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413. [PMID: 32865377] SolomonSDet al; DELIVER Trial Committees and Investigators. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089. [PMID: 36027570] SolomonSDet al; PARAGON-HF Investigators and Committees. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609. [PMID: 31475794] VelazquezEJet al; PIONEER-HF Investigators. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539. [PMID: 30415601] ZannadFet al; COMMANDER HF Investigators. Rivaroxaban in patients with heart failure, sinus rhythm, and coronary disease. N Engl J Med. 2018;379:1332. [PMID: 30146935] |