| ESSENTIALS OF DIAGNOSIS | ||
|
General Considerations
Asthma is a common disease, affecting approximately 8-10% of the population. It is slightly more common in male children (younger than 14 years) and in female adults. There is a genetic predisposition to asthma. Prevalence, hospitalizations, and fatal asthma have all increased in the United States over the past 20 years. Each year, approximately 10 million office visits, 1.8 million emergency department visits, and more than 3500 deaths in the United States are attributed to asthma. Hospitalization rates are highest among Black persons and children, and death rates are consistently highest among Black persons aged 15-24 years. The Global Initiative for Asthma (GINA) report was updated in 2023 to provide a comprehensive resource that addresses asthma diagnosis, assessment, management, and evidence-based recommendations.
Definition & Pathogenesis
Asthma is a chronic disorder of the airways that results in an array of respiratory symptoms and signs and is characterized by variable levels of airway expiratory obstruction and hyperresponsiveness. The most common pathogeneses of asthma include airway inflammation with eosinophils, neutrophils, and lymphocytes (especially T cells); goblet cell hyperplasia; plugging of small airways with mucus; collagen deposition beneath the basement membrane; bronchial smooth muscle hypertrophy; airway edema; mast cell activation; and denudation of airway epithelium. The pathophysiology of asthma is heterogeneous, but a division into T2-high and T2-low endotypes (marked by high and low levels, respectively, of classic Th2 cytokines such as interleukin [IL]-4, IL-5, and IL-13) has been shown to be important in the selection of targeted biologic therapies.
Many clinical phenotypes of asthma have been identified. The most common is allergic asthma, which usually begins in childhood and is associated with other allergic diseases such as eczema, allergic rhinitis, or food allergy. Exposure of sensitive patients to inhaled allergens may cause symptoms immediately (early asthmatic response) or 4-6 hours after allergen exposure (late asthmatic response). Common allergens include house dust mites (often found in pillows, mattresses, upholstered furniture, carpets, and drapes), cockroaches, cat dander, and seasonal pollens. Substantially reducing exposure reduces pathologic findings and clinical symptoms. Allergic asthma, late-onset T2-high asthma, and aspirin/NSAID-associated respiratory disease are T2-high phenotypes. T2-low asthma phenotypes include nonallergic asthma, which tends to occur in adults and be marked by neutrophilic inflammation and variable response to standard therapies. Asthma with persistent airflow limitation is thought to be due to airway remodeling. Asthma with obesity refers to prominent respiratory symptoms in patients with obesity with little airway inflammation.
Nonspecific precipitants of asthma include upper respiratory tract infections, rhinosinusitis, postnasal drip, aspiration, gastroesophageal reflux, changes in the weather, stress, and exercise. Exposure to products of combustion (eg, tobacco, methamphetamines, diesel fuel, and other agents) increases asthma symptoms and the need for medications and reduces lung function. Air pollution (increased air levels of respirable particles, ozone, SO2, and NO2) precipitates asthma symptoms and increases emergency department visits and hospitalizations. Selected individuals may experience asthma symptoms after exposure to aspirin (aspirin-exacerbated respiratory disease), NSAIDs, or tartrazine dyes. Other medications may precipitate asthma symptoms (see Table 9-23). Occupational asthma is triggered by various agents in the workplace and may occur weeks to years after initial exposure and sensitization. Women may experience catamenial asthma at predictable times during the menstrual cycle. Exercise-induced bronchoconstriction begins during exercise or within 3 minutes after its end, peaks within 10-15 minutes, and then resolves by 60 minutes. This phenomenon is thought to be a consequence of the airways' warming and humidifying an increased volume of expired air during exercise. Cough-variant asthma has cough instead of wheezing as the predominant symptom of bronchial hyperreactivity.
Clinical Findings
Symptoms and signs vary widely among patients as well as within individuals over time. The level of asthma control is assessed by the frequency of day and nighttime symptoms and need for reliever medications as listed in Table 9-1. Assessing Asthma Control.
Table 9-1. Assessing asthma control.| Components of Asthma Control | Classification of Asthma Control | ||
|---|---|---|---|
| Well Controlled | Partly Controlled | Not Controlled | |
| Daytime asthma symptoms >2 ×/week |
None of these components within past 4 weeks |
1 or 2 of these components within past 4 weeks |
3 or 4 of these components within past 4 weeks |
| Nighttime awakenings due to asthma | |||
| Interference with normal activity due to asthma | |||
| Reliever medication needed for asthma symptoms >2 ×/week | |||
Adapted from National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. National Institutes of Health Pub. No. 08-4051. Bethesda, MD, 2007, and Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention 2022. Available at http://ginasthma.org
A. Symptoms and Signs
Asthma is characterized by episodic wheezing, shortness of breath, chest tightness, and cough. Symptoms vary over time and in intensity and are often worse at night or in the early morning. Asthma symptoms may occur spontaneously or be precipitated or exacerbated by many different triggers, as discussed above. The following features decrease the likelihood that respiratory symptoms are due to asthma: isolated cough with no other symptoms, chronic sputum production, chest pain, and shortness of breath with paresthesias.
Some physical examination findings increase the probability of asthma. Nasal mucosal swelling, increased secretions, and polyps are often seen in patients with allergic asthma. Eczema, atopic dermatitis, or other allergic skin disorders may also be present. Wheezing, a prolonged expiratory phase, or both during normal breathing are suggestive of airflow obstruction; wheezing during forced expiration does not. Chest examination may be normal between exacerbations in patients with mild asthma. During severe asthma exacerbations, airflow may be too limited to produce wheezing, and the only diagnostic clue on auscultation may be globally reduced breath sounds with prolonged expiration. Hunched shoulders and use of accessory muscles of respiration suggest an increased work of breathing.
B. Laboratory Findings
ABG measurements may be normal during a mild asthma exacerbation, but respiratory alkalosis (with low PaCO2) and an increase in the alveolar-arterial oxygen difference (A-a-DO2) are common. During severe exacerbations, hypoxemia develops and the PaCO2 returns to normal due to retention. The combination of an increased PaCO2 and respiratory acidosis may indicate impending respiratory failure and the need for mechanical ventilation.
C. Pulmonary Function Testing
Pulmonary function testing with either spirometry or PEF measurements are important for the diagnosis and management of patients with asthma.
Important spirometry measurements include FEV1, FVC, and FEV1/FVC before and after the administration of a short-acting bronchodilator (eFigure 9-1). These measurements help determine the presence and extent of airflow obstruction and whether it is immediately reversible. Airflow obstruction is indicated by a reduced FEV1/FVC ratio, generally below 0.7 or the lower limit of normal. Significant reversibility of airflow obstruction was previously defined by an increase of 12% or more and 200 mL in FEV1 or FVC after inhaling a short-acting bronchodilator. Based on 2022 guidelines, bronchodilator response is defined by an increase in FEV1 or FVC of greater than 10% relative to the predicted value. A positive bronchodilator response supports the diagnosis of asthma, but a lack of responsiveness does not preclude response to a clinical trial of bronchodilator therapy. Severe airflow obstruction results in significant air trapping, with an increase in residual volume and consequent reduction in FVC, resulting in a pattern that may mimic a restrictive ventilatory defect.
Bronchoprovocation testing with inhaled histamine or methacholine may be useful when asthma is suspected despite nondiagnostic spirometry. Bronchial provocation is not recommended if the FEV1 is less than 65% of predicted. A positive methacholine test is defined as a fall in the FEV1 of 20% or more at exposure to a methacholine concentration of less than or equal to 8 mg/mL. A negative methacholine test has a negative predictive value for asthma of 95%. Exercise challenge testing may be useful in patients with symptoms of exercise-induced bronchospasm.
PEF meters are handheld devices designed as personal monitoring tools. PEF monitoring can establish peak flow variability, quantify asthma severity, and provide both patient and clinician with objective measurements on which to base treatment decisions. There are conflicting data about whether measuring PEF improves asthma outcomes but doing so is recommended to help confirm the diagnosis of asthma, to improve asthma control in patients with poor perception of airflow obstruction, and to identify environmental and occupational causes of symptoms. Predicted values for PEF vary with age, height, and sex but are poorly standardized. Comparison with reference values is less helpful than comparison with the patient's own baseline. PEF shows diurnal variation; it is generally lowest on first awakening and highest several hours before the midpoint of the waking day. PEF should be measured in the morning before the administration of a bronchodilator and in the afternoon after taking a bronchodilator. A 20% change in PEF values from morning to afternoon or from day to day suggests inadequately controlled asthma. PEF values less than 200 L/minute indicate severe airflow obstruction.
D. Additional Testing
Routine CXRs in patients with asthma are usually normal or only show hyperinflation. Other findings may include bronchial wall thickening and diminished peripheral lung vascular markings. Chest imaging is indicated when pneumonia, which may mimic asthma, or a complication of asthma such as pneumothorax is suspected.
Skin or in vitro testing, including total serum IgE and allergen-specific IgE, to assess sensitivity to environmental allergens can identify atopy in patients with persistent asthma who may benefit from therapies directed at their allergic diathesis. Evaluations for paranasal sinus disease or gastroesophageal reflux should be considered in patients with persistent, severe, or refractory asthma symptoms. An absolute eosinophil count can identify patients eligible for anti-IL-5 therapy to manage eosinophilic airway disease.
Noninvasive assessment of underlying airway inflammation through measurement of eosinophilia in induced sputum, or fractional nitric oxide concentration in exhaled breath condensates (FENO), offers the promise of improved diagnosis and treatment strategies. Adjusting corticosteroid dose to minimize sputum eosinophilia appears to reduce the frequency of exacerbations compared with conventional clinical management, but data are conflicting regarding the impact of FENO on asthma outcomes.
Complications
Complications of asthma include exhaustion, dehydration, airway infection, tussive syncope, and, rarely, pneumothorax. Acute hypercapnic and hypoxemic respiratory failure occurs in severe disease.
Differential Diagnosis
Patients who have atypical symptoms or poor response to therapy may have one of several conditions that mimic asthma. Upper airway disorders that mimic asthma include vocal fold paralysis, vocal fold dysfunction syndrome, narrowing of the supraglottic airway, and laryngeal masses or dysfunction. Lower airway disorders include foreign body aspiration, tracheal masses or narrowing, tracheobronchomalacia, airway edema (eg, angioedema or inhalation injury), nonasthmatic COPD (chronic bronchitis or emphysema), bronchiectasis, allergic bronchopulmonary aspergillosis (mycosis), cystic fibrosis, eosinophilic pneumonia, hypersensitivity pneumonitis, sarcoidosis, and bronchiolitis obliterans. A systemic vasculitis with pulmonary involvement may have an asthmatic component, such as eosinophilic granulomatosis with polyangiitis. Cardiac disorders include HF (“cardiac asthma”), and pulmonary hypertension. Psychiatric causes include conversion disorders (“functional” asthma), emotional laryngeal wheezing, or episodic laryngeal dyskinesis. Rarely, Münchausen syndrome or malingering may explain a patient's symptom presentation.
Approach to Management
Personalized asthma management is a continuous cycle that involves assessment, treatment adjustment, and periodic review with the goals of optimal symptom control; minimization of future risks, including exacerbations; and prevention of asthma-related deaths, as recommended in the updated 2023 GINA report for asthma. Asthma assessment includes the level of asthma control, risk factors for exacerbations, asthma severity, treatment adjustment, and periodic lung function testing.
1. Asthma Control
Level of control is assessed by evaluating symptoms. Patients are asked about their past 4 weeks including frequency of symptoms (days per week), awakening from sleep, and use of reliever therapy (short-acting beta-agonist (SABA), inhaled corticosteroid (ICS)-formoterol, or ICS-SABA) for symptom relief (Table 9-1. Assessing Asthma Control). Patients should also be asked about activity limitation.
2. Risk Factors for Exacerbations
Poor symptom control increases risk of exacerbations. Other risk factors include more than one exacerbation in the previous year; inadequate inhaled corticosteroid (ICS) use (due to under-treatment, poor adherence, or incorrect inhaler technique); and other comorbidities, such as chronic sinusitis, GERD, obesity, and smoking.
3. Asthma Severity
Severity is evaluated retrospectively from the level of treatment needed to control symptoms and exacerbations. Table 9-2. Step Therapy in Personalized Asthma Management Plan describes the step therapy in a personalized asthma management plan. Typically, mild asthma responds to Step 1 or 2 treatments, moderate asthma to Step 3 treatment, and severe asthma to Step 4 or 5 treatments. It is important to distinguish between uncontrolled and severe asthma in patients who are using Step 4 or Step 5 treatments. The clinician must assess inhaler technique, medication adherence, comorbidities such as obstructive sleep apnea or GERD, and ongoing exposure to allergens as causes of poor asthma control (“uncontrolled” asthma). If the patient still requires Step 4 or 5 therapy after these issues have been addressed, then the patient has “severe” asthma and should be referred to a pulmonary or asthma specialist. Serial lung function testing is beneficial at time of diagnosis, 3-6 months after treatment initiation, and periodically thereafter but is not necessarily needed at every visit.
Table 9-2. Step therapy in personalized asthma management plan.| Preferred | Alternative | |
|---|---|---|
| Steps 1 and 2 | Low-dose ICS-formoterol as needed or Low-dose ICS daily and SABA as needed or Concomitant ICS and SABA as needed | LTRA daily and SABA as needed or Cromolyn or Nedocromil or Zileuton or Theophylline and SABA as needed |
| Step 3 | Combination low-dose ICS plus formoterol daily and as needed | Medium-dose ICS daily and SABA as needed or Low-dose ICS-LABA daily or Low-dose ICS plus LAMA daily or Low-dose ICS plus LTRA and SABA as needed or Low-dose ICS daily plus theophylline or zileuton and SABA as needed |
| Step 4 | Combination medium-dose ICS-formoterol daily and as needed | Medium-dose ICS-LABA daily or Medium-dose ICS plus LAMA daily and SABA as needed or Medium-dose ICS plus LTRA daily or Medium-dose ICS plus zileuton daily and SABA as needed or Medium-dose ICS plus theophylline daily |
| Step 5 | Medium-high dose ICS-LABA plus LAMA and SABA as needed | Medium-high dose ICS-LABA daily or High-dose ICS plus LTRA and SABA as needed |
| Step 6 | High-dose ICS-LABA plus oral systemic corticosteroids plus SABA as needed |
4. Treatment Adjustment
The goals of asthma therapy are to minimize chronic symptoms that interfere with normal activity (including exercise), prevent recurrent exacerbations, reduce or eliminate the need for emergency department visits or hospitalizations, and maintain normal or near-normal pulmonary function. A multidisciplinary approach using pharmacologic and nonpharmacologic strategies is best to address disease pathogenesis and modifiable risk factors. Pharmacologic agents that satisfy the patient's expectations of asthma care with the fewest adverse events should be prescribed. Management should include stepping up therapy if asthma remains uncontrolled despite adherence and good inhaler technique and stepping down if asthma is well controlled to find the minimum effective therapeutic dose. Nonpharmacologic interventions include increasing physical activity and breathing exercises. Significant reduction in exposure to nonspecific airway irritants in all patients or to inhaled allergens in atopic patients may reduce symptoms and medication needs. Comorbid conditions that impair asthma management, such as smoking, rhinosinusitis, GERD, obesity, and obstructive sleep apnea, should be identified and treated. The asthma plan of care, level of symptom control, and patient satisfaction should be reviewed on a periodic basis and facilitated by guided patient self-management education and skills training. Self-management includes self-monitoring of symptoms or peak flow; a written action plan; and regular review of asthma control, treatment, and skills with a health care professional.
Treatment
A. Pharmacologic Agents
Asthma medications can be divided into three categories: (1) long-term controller medications (Table 9-3. Long-Term Controller Medications for Asthma) used long-term to reduce airway inflammation, symptoms, and risk of future exacerbations, (2) reliever medications (Table 9-4. Reliever Medications for Asthma) used on an as-needed basis to relieve breakthrough symptoms, and (3) add-on therapies for severe asthma. Table 9-2. Step Therapy in Personalized Asthma Management Plan shows a personalized management plan for asthma to control symptoms and minimize future risk.
Table 9-3. Long-term controller medications for asthma.| Medication | Dosage Form | Adult Dose | Comments |
|---|---|---|---|
| Inhaled Corticosteroids (ICS) | (See Table 9-5. Estimated Clinically Comparable Daily Dosages for Inhaled Corticosteroids for Adults with Asthma) | ||
| Systemic Corticosteroids | (Applies to all three corticosteroids) | ||
| Methylprednisolone | 2-, 4-, 6-, 8-, 16-, 32-mg tablets | 40-60 mg |
|
| Prednisolone | 5-mg tablets; 5 mg/5 mL, 15 mg/5 mL oral solution | 40-60 mg | |
| Prednisone | 1-, 2.5-, 5-, 10-, 20-, 50-mg tablets; 5 mg/mL oral solution | 7.5-60 mg | |
| Inhaled LABA | Should not be used for symptom relief or exacerbations. Use with ICS. | ||
| Formoterol | Inhalation: 20 mcg/2 mL nebulizer (DPI discontinued by FDA in United States) | 20 mcg every 12 hours |
|
| Salmeterol | DPI: 50 mcg/actuation | 1 blister every 12 hours | |
| Combined Medication | |||
| Budesonide/formoterol | HFA MDI: 80 mcg/4.5 mcg 160 mcg/4.5 mcg | 2 inhalations twice daily; dose depends on severity of asthma |
|
| Fluticasone/salmeterol | DPI: 100 mcg/50 mcg 250 mcg/50 mcg 500 mcg/50 mcg HFA: 45 mcg/21 mcg 115 mcg/21 mcg 230 mcg/21 mcg | 1 inhalation twice daily; dose depends on severity of asthma |
|
| Fluticasone furoate/vilanterol | DPI: 100 mcg/25 mcg or 200 mcg/25 mcg per blister | 1 puff inhaled daily |
|
| Mometasone/formoterol | 100 mcg/5 mcg/spray 200 mcg/5 mcg/spray | 2 inhalations twice daily | |
| Cromolyn and Nedocromil | |||
| Cromolyn | MDI: 0.8 mg/puff Nebulizer: 20 mg/ampule | 2 puffs four times daily 1 ampule four times daily |
|
| Nedocromil | MDI: 1.75 mg/puff | 2 puffs four times daily | |
| Inhaled Long-Acting Anticholinergic | Should not be used for symptom relief or exacerbations. Use with ICS. | ||
| Tiotropium | DPI: 18 mcg/blister | 1 blister daily | |
| Leukotriene Receptor Antagonists | |||
| Montelukast | 4- or 5-mg chewable tablet; 10-mg tablet | 10 mg daily at bedtime |
|
| Zafirlukast | 10- or 20-mg tablet | 20-mg tablet twice daily |
|
| 5-Lipoxygenase Inhibitor | |||
| Zileuton | 600-mg tablet | 600 mg four times daily |
|
| Methylxanthines | |||
| Theophylline | Liquids, sustained-release tablets, and capsules | Starting dose: 10 mg/kg/day up to 300 mg maximum Usual maximum dose: 800 mg/day |
|
| Monoclonal Antibodies | |||
| Omalizumab | Subcutaneous injection | Dependent on pretreatment IgE level; up to 375 mg every 2 weeks |
|
| Mepolizumab | Subcutaneous injection | 100 mg every 4 weeks |
|
| Reslizumab | Intravenous injection | 3 mg/kg every 4 weeks |
|
| Benralizumab | Subcutaneous injection | 30 mg every 4 weeks for 3 doses, then every 8 weeks |
|
| Dupilumab | Subcutaneous injection | 200 or 300 mg every 2 weeks |
|
AEC, absolute eosinophil count; DPI, dry powder inhaler; EIB, exercise-induced bronchospasm; FENO, fractional exhaled nitric oxide; HFA, hydrofluoroalkane; LABA, long-acting beta-2-agonist; MDI, metered-dose inhaler; SABA, short-acting beta-2-agonist.
| Medication | Dosage Form | Adult Dose | Comments | |
|---|---|---|---|---|
| Inhaled Short-Acting Beta-2-Agonists (SABA) | ||||
| Albuterol CFC | MDI: 90 mcg/puff, 200 puffs/canister | 2 puffs 5 minutes before exercise 2 puffs every 4-6 hours as needed |
| |
| Albuterol HFA | MDI: 90 mcg/puff, 200 puffs/canister | 2 puffs 5 minutes before exercise 2 puffs every 4-6 hours as needed | ||
| Pirbuterol CFC | MDI: 200 mcg/puff, 400 puffs/canister | 2 puffs 5 minutes before exercise 2 puffs every 4-6 hours as needed | ||
| Levalbuterol HFA | MDI: 45 mcg/puff, 200 puffs/canister | 2 puffs 5 minutes before exercise 2 puffs every 4-6 hours as needed | ||
| Albuterol | Nebulizer solution: 0.63 mg/3 mL 1.25 mg/3 mL 2.5 mg/3 mL 5 mg/mL (0.5%) | 1.25-5 mg in 3 mL of saline every 4-8 hours as needed |
| |
| Levalbuterol (R-albuterol) | Nebulizer solution: 0.31 mg/3 mL 0.63 mg/3 mL 1.25 mg/0.5 mL 1.25 mg/3 mL | 0.63-1.25 mg every 8 hours as needed |
| |
| Anticholinergics | ||||
| Ipratropium HFA | MDI: 17 mcg/puff, 200 puffs/canister | 2-3 puffs every 6 hours |
| |
Nebulizer solution: 0.25 mg/mL (0.025%) | 0.25 mg every 6 hours | |||
| Ipratropium with albuterol | MDI: 18 mcg/puff of ipratropium bromide and 90 mcg/puff of albuterol, 200 puffs/canister | 2-3 puffs every 6 hours | ||
Nebulizer solution: 0.5 mg/3 mL ipratropium bromide and 2.5 mg/3 mL albuterol | 3 mL every 4-6 hours |
| ||
| Anti-Inflammatory Relievers (Low-dose ICS and Rapid Acting Bronchodilator) | ||||
| ICS-Albuterol | Albuterol/Budesonide MDI: 90 mcg/80 mcg per actuation | 2 puffs every 4 hours as needed | Max 12 puffs/day | |
| Budesonide/Formoterol | MDI: 80 mcg/4.5 mcg per actuation | 1-2 puffs every 4 hours as needed |
| |
| Beclomethasone/Formoterol | MDI: 100 mcg/6 mcg per actuation | 1-2 puffs every 4 hours as needed | Max 8 inhalation per day | |
| Systemic Corticosteroids | ||||
| Methylprednisolone | 2-, 4-, 6-, 8-, 16-, 32-mg tablets | 40-60 mg/day as single or 2 divided doses |
| |
| Prednisolone | 5-mg tablets; 5 mg/5 mL, 15 mg/5 mL oral solution | 40-60 mg/day as single or 2 divided doses | ||
| Prednisone | 1-, 2.5-, 5-, 10-, 20-, 50-mg tablets; 5 mg/mL oral solution | 40-60 mg/day as single or 2 divided doses | ||
| Methylprednisolone acetate | Repository injection: 40 mg/mL 80 mg/mL | 240 mg intramuscularly once |
| |
CFC, chlorofluorocarbon; EIB, exercise-induced bronchospasm; HFA, hydrofluoroalkane; MDI, metered-dose inhaler.
Adapted from National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. National Institutes of Health Pub. No. 08-4051. Bethesda, MD, 2007.
| Medication | Low Daily Dose | Medium Daily Dose | High Daily Dose |
|---|---|---|---|
Beclomethasone dipropionate HFA 40 or 80 mcg/puff |
80-240 mcg |
>240-480 mcg |
>480 mcg |
Budesonide dipropionate DPI 90, 180, or 200 mcg/inhalation |
180-400 mcg |
>400-800 mcg |
>800 mcg |
Flunisolide 250 mcg/puff |
500-1000 mcg |
>1000-2000 mcg |
>2000 mcg |
Flunisolide HFA 80 mcg/puff |
320 mcg |
>320-640 mcg |
>640 mcg |
Fluticasone propionate HFA/MDI: 44, 110, or 220 mcg/puff DPI: 50, 100, or 250 mcg/inhalation |
88-264 mcg 100-300 mcg |
>264-440 mcg >300-500 mcg |
>440 mcg >500 mcg |
Mometasone furoate DPI 200 mcg/puff |
200 mcg |
400 mcg |
>400 mcg |
Triamcinolone acetonide 75 mcg/puff |
300-750 mcg |
>750-1500 mcg |
>1500 mcg |
DPI, dry powder inhaler; HFA, hydrofluoroalkane; MDI, metered-dose inhaler.
- The most important determinant of appropriate dosing is the clinician's judgment of the patient's response to therapy. Most of clinical benefit from inhaled corticosteroid therapy is seen at low doses; responsiveness varies among patients.
- Potential drug interactions: Several inhaled corticosteroids, including fluticasone, budesonide, and mometasone, are metabolized in the GI tract and liver by CYP 3A4 isoenzymes. Potent inhibitors of CYP 3A4, such as ritonavir and ketoconazole, have the potential for increasing systemic concentrations of these inhaled corticosteroids by increasing oral availability and decreasing systemic clearance. Some cases of clinically significant Cushing syndrome and secondary adrenal insufficiency have been reported.
Adapted from National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. National Institutes of Health Pub. No. 08-4051. Bethesda, MD, 2007, and Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2019. (Available from: www.ginasthma.org.)
Most asthma medications are administered by inhalation or oral dosing. Inhalation of an appropriate agent results in a more rapid onset of pulmonary effects and fewer systemic effects compared with the oral dose required to achieve the same effect. Proper inhaler technique and the use of an inhalation chamber (a “spacer”) with metered-dose inhalers (MDIs) decrease oropharyngeal drug deposition and improve drug delivery to the lung. Nebulizer therapy is reserved for patients who are acutely ill and those who cannot use inhalers because of difficulties with coordination, understanding, or cooperation.
1. Inhaled Corticosteroids
Inhaled corticosteroids (ICS) are essential controller medications (Tables 9-4. Reliever Medications for Asthma and 9-5. Estimated Clinically Comparable Daily Dosages for Inhaled Corticosteroids for Adults with Asthma). Once the diagnosis of asthma is made, early initiation of ICS therapy leads to a greater improvement in lung function than delayed therapy. Prescribing as-needed or daily controller ICS at the start of asthma therapy conveys a message to patients that both symptom control and risk reduction are the mainstays of asthma treatment. The most important determinants of medication choice, device, and dose are a patient's symptoms and risk factors, along with practical issues (such as cost and delivery mechanism). ICS dosages are classified as low-, medium-, and high-dose strengths, but low-dose ICS provides clinical benefit and is sufficient for most patients with asthma. Dosages for ICS vary depending on the specific agent and delivery device (Table 9-5. Estimated Clinically Comparable Daily Dosages for Inhaled Corticosteroids for Adults with Asthma). For patients who require high-dose ICS to achieve adequate symptom control, the dose of inhaled corticosteroid should be decreased after 3 months of good control to the lowest dose that preserves symptom control and minimizes exacerbation risk.
Concomitant use of an MDI and an inhalation chamber coupled with mouth washing after ICS use decreases systemic absorption and local side effects (cough, dysphonia, oropharyngeal candidiasis). Dry powder inhalers (DPIs) are not used with an inhalation chamber. Systemic effects (adrenal suppression, osteoporosis, skin thinning, easy bruising, and cataracts) may occur with high-dose ICS therapy. Combination inhalers with an ICS and a long-acting beta-2-agonist (LABA) offer convenient treatment of asthma. The GINA report recommends low-dose inhaled corticosteroid/formoterol as its preferred agent due to clinical evidence but notes that its cost and availability in different countries must be taken into consideration. Budesonide/formoterol is listed as a WHO essential medication.
2. Beta-Adrenergic Agonists
Beta-agonists are divided into SABAs and LABAs. SABAs (Table 9-4. Reliever Medications for Asthma), including agents such as albuterol, levalbuterol, bitolterol, pirbuterol, and terbutaline, are mainstays of reliever or rescue therapy for asthma patients. There is no convincing evidence to support the use of one agent over another. All asthmatics should have immediate access to a bronchodilator, preferably SABA, because they are the most effective bronchodilators during exacerbations and provide immediate relief of symptoms. Alternatively, GINA has proposed the combination of low-dose glucocorticoid plus formoterol, a fast-acting LABA, or combination of low dose inhaled corticosteroid plus SABA, as symptom relief on as needed basis instead. Administration before exercise effectively prevents exercise-induced bronchoconstriction.
Inhaled SABA therapy is as effective as oral or parenteral beta-agonist therapy in relaxing airway smooth muscle and improving acute asthma and offers the advantages of rapid onset of action (less than 5 minutes) with fewer systemic side effects. Beta-2-selective agents may produce less cardiac stimulation than those with mixed beta-1 and beta-2 activities, although clinical trials have not consistently demonstrated this finding. Repetitive administration produces incremental bronchodilation. One or two inhalations of a SABA from an MDI are usually sufficient for mild to moderate symptoms. Severe exacerbations frequently require higher doses: 6-12 puffs every 30-60 minutes of albuterol by MDI with an inhalation chamber or 2.5 mg by nebulizer provide equivalent bronchodilation. Administration by nebulization does not offer more effective delivery than MDIs used correctly but does provide higher doses. With most SABAs, the recommended dose by nebulizer for acute asthma (albuterol, 2.5 mg) is 25-30 times that delivered by a single activation of the MDI (albuterol, 0.09 mg). This difference suggests that standard dosing of inhalations from an MDI may be insufficient in the setting of an acute exacerbation. Independent of dose, nebulizer therapy may be more effective in patients who are unable to coordinate inhalation of medication from an MDI because of age, agitation, or severity of the exacerbation.
GINA recommends against SABA-only treatment of asthma in adults or adolescents and does not recommend scheduled daily use of SABAs. Although SABA is effective as a quick relief medication, patients who are treated with SABA alone are at increased risk for asthma-related death and urgent health care even if their symptoms are controlled. Increased use (more than one canister a month) or lack of expected effect indicates diminished asthma control and the need for additional long-term controller therapy.
LABAs provide bronchodilation for up to 12 hours after a single dose. Salmeterol and formoterol are LABAs available for asthma in the United States. Olodaterol, indacaterol, and arformoterol are approved in the United States for COPD but not for asthma. In combination with an ICS, they are indicated for maintenance therapy of asthma. LABAs should not be used as monotherapy because they have no anti-inflammatory effect and because monotherapy has been associated with a small but significant increased risk of severe or fatal asthma attacks in two large studies. Based on clinical trial data in patients with mild asthma, combination inhalers containing formoterol and low-dose budesonide are the preferred option, which have demonstrated a 64% reduction in severe exacerbations compared with SABA-only treatment and noninferiority for severe exacerbations compared to low-dose ICS alone.
3. Systemic Corticosteroids
Systemic corticosteroids (oral prednisone or prednisolone or parenteral methylprednisolone) are most effective in achieving prompt control of asthma during acute exacerbations. Systemic corticosteroids are effective as primary treatments for patients with moderate to severe asthma exacerbations and for patients with exacerbations that do not respond promptly and completely to inhaled SABA therapy. These medications speed the resolution of airflow obstruction and reduce the rate of relapse. Delays in administering corticosteroids may result in progressive impairment. Therefore, patients with moderate to severe asthma should be prescribed oral corticosteroids so they are available for early, at-home administration, when needed. The minimal effective dose of systemic corticosteroids for asthma patients has not been identified. Outpatient prednisone “burst” therapy is 0.5-1 mg/kg/day (typically 40-60 mg) in 1-2 doses for 3-7 days. Severe exacerbations requiring hospitalization typically require 1 mg/kg of prednisone or methylprednisolone every 6-12 hours for 48 hours or until the FEV1 (or PEF rate) returns to 50% of predicted (or 50% of baseline). The dose is then decreased to 0.5 mg/kg/day until the PEF reaches 70% of predicted or personal best. No clear advantage has been found for higher doses of corticosteroids. It may be prudent to administer corticosteroids intravenously to critically ill patients to avoid concerns about altered GI absorption.
In patients with refractory, poorly controlled asthma, or patients with frequent exacerbations despite optimized inhaler therapy, systemic corticosteroids may be required for the long-term suppression of symptoms. Repeated efforts should be made to reduce the dose to the minimum needed to control symptoms. Concurrent treatment with calcium supplements and vitamin D should be initiated to prevent corticosteroid-induced bone mineral loss with long-term administration. Bone mineral density testing after 3 or more months of cumulative systemic corticosteroid exposure can guide the use of bisphosphonates for treatment of steroid-induced osteoporosis. Rapid discontinuation of systemic corticosteroids after long-term use may precipitate adrenal insufficiency.
4. Anticholinergics
Anticholinergic agents reverse vagally mediated bronchospasm but not allergen- or exercise-induced bronchospasm. They may decrease mucous gland hypersecretion. Both short-acting muscarinic antagonists (SAMAs) and long-acting muscarinic antagonists (LAMAs) are available. Ipratropium bromide, a SAMA, is less effective than SABA for relief of acute bronchospasm but is the inhaled drug of choice for patients with intolerance to SABA or bronchospasm due to beta-blocker medications. Ipratropium bromide reduces the rate of hospital admissions when added to inhaled SABAs in patients with moderate to severe asthma exacerbations. Although LAMAs have long been the cornerstone of therapy for COPD, their role in asthma continues to evolve. Studies have shown that the addition of tiotropium to medium-dose inhaled corticosteroid and salmeterol improves lung function and reduces the frequency of asthma exacerbations.
5. Leukotriene Modifiers
Leukotrienes are potent mediators that contribute to airway obstruction and asthma symptoms by contracting airway smooth muscle, increasing vascular permeability and mucous secretion, and attracting and activating airway inflammatory cells. Zileuton is a 5-lipoxygenase inhibitor that decreases leukotriene production, and zafirlukast and montelukast are cysteinyl leukotriene receptor antagonists. In RCTs, these agents caused modest improvements in lung function and reductions in asthma symptoms and lessened the need for SABA rescue therapy. These agents are less effective than inhaled corticosteroid for exacerbation reduction or as first-line controller therapy but may be considered as alternatives in patients with asthma who are unable to take inhaled corticosteroid or who experience undesirable corticosteroid side effects.
6. Monoclonal Antibody Agents
Asthmatic patients who require monoclonal antibody therapies should be evaluated by either a pulmonologist or allergist experienced in their use. Omalizumab is a recombinant antibody that binds IgE without activating mast cells. Clinical trials in patients with moderate to severe asthma and elevated serum IgE levels have found that omalizumab, when administered subcutaneously every 2-4 weeks, reduced the need for corticosteroids. Three other IL-5 antagonist monoclonal antibodies (anti IL-5/5R) are approved for the treatment of severe asthma with peripheral blood eosinophilia that has not responded to standard treatments: reslizumab (administered intravenously every 4 weeks), mepolizumab (administered subcutaneously every 4 weeks), and benralizumab (administered subcutaneously every 4-8 weeks). Dupilumab is a monoclonal antibody (anti-IL-4Rα) that, when administered subcutaneously every 2 weeks, inhibits overactive signaling of IL-4 and IL-13.
7. Phosphodiesterase Inhibitor
Theophylline provides mild bronchodilation in asthmatic patients. It also has anti-inflammatory and immunomodulatory properties, enhances mucociliary clearance, and strengthens diaphragmatic contractility. Sustained-release theophylline preparations are effective in controlling nocturnal symptoms and as added therapy in patients with moderate or severe persistent asthma whose symptoms are inadequately controlled by inhaled corticosteroids. Low-dose sustained-release theophylline is included as a less effective option in Step 3 treatment. Neither theophylline nor aminophylline is recommended for therapy of acute asthma exacerbations.
Theophylline has a notably narrow therapeutic-toxic range. At therapeutic doses, potential adverse effects include insomnia, aggravation of dyspepsia and gastroesophageal reflux, and urination difficulties in men with prostatic hyperplasia. Dose-related toxicities include nausea, vomiting, tachyarrhythmias, headache, seizures, hyperglycemia, and hypokalemia. Theophylline serum levels are highly variable due to many factors that alter drug absorption, significant individual differences in metabolism, and multiple drug-drug interactions. Therefore, serum concentrations need to be monitored closely.
Theophylline and its intravenous formulation, aminophylline, are not recommended for therapy of asthma exacerbations. Aminophylline has clearly been shown to be less effective than SABAs when used as single-drug therapy for acute asthma; it adds little except toxicity to the acute bronchodilator effects achieved by nebulized metaproterenol alone. Patients with exacerbations who are currently taking theophylline should have its serum concentration measured to exclude theophylline toxicity.
8. Mediator Inhibitors
Cromolyn sodium and nedocromil are long-term control medications that prevent asthma symptoms and improve airway function in patients with mild persistent or exercise-induced asthma. These agents modulate mast cell mediator release and eosinophil recruitment and inhibit both early and late asthmatic responses to allergen challenge and exercise-induced bronchospasm. The clinical response to these agents is less predictable than to inhaled corticosteroids. Both agents have excellent safety profiles.
B. Desensitization
Immunotherapy for specific allergens may be considered in selected asthma patients who have exacerbations when exposed to allergens to which they are sensitive and when unresponsive to environmental control measures or other therapies. Studies show a reduction in asthma symptoms in patients treated with single-allergen immunotherapy. Because of the risk of immunotherapy-induced bronchoconstriction, it should be administered only in a setting where such complications can be immediately treated.
C. Vaccination
All adult patients with asthma should receive appropriate pneumococcal, influenza, and COVID-19 vaccination. (See Community Acquired Pneumonia: Prevention for full description.) The use of the intranasal live attenuated influenza vaccine may be associated with asthma exacerbations in young children.
Treatment of Asthma Exacerbations
Asthma expert panels including GINA and National Asthma Education and Prevention Program iterate the importance of patient education in early recognition and intervention of asthma exacerbations. An asthma action plan developed by care providers can assist the patient in their daily routine care and outline worsening signs or symptoms that suggest the need for health care evaluation. Symptoms of exacerbations include progressive breathlessness, increasing chest tightness, decreased peak flow, and lack of improvement after SABA therapy (Table 9-6. Evaluation and Classification of Severity of Asthma Exacerbations). Most instances of uncontrolled asthma are mild and may be managed by patients at home with self-management plans. More severe exacerbations with persistent or worsening symptoms require evaluation and management by a health care provider who can assess the patient's exacerbation severity, respiratory status, and risk factors for asthma related mortality and direct management accordingly (Figure 9-1).
Table 9-6. Evaluation and classification of severity of asthma exacerbations.| Mild | Moderate | Severe | Respiratory Arrest Imminent | |
|---|---|---|---|---|
| Symptoms | ||||
| Breathlessness | While walking | At rest, limits activity | At rest, interferes with conversation | While at rest, mute |
| Talks in | Sentences | Phrases | Words | Silent |
| Alertness | May be agitated | Usually agitated | Usually agitated | Drowsy or confused |
| Signs | ||||
| Respiratory rate | Increased | Increased | Often >30/minute | >30/minute |
| Body position | Can lie down | Prefers sitting | Sits upright | Unable to recline |
| Use of accessory muscles, suprasternal retractions | Usually not | Commonly | Usually | Paradoxical thoracoabdominal movement |
| Wheezing | Moderate, often only end expiratory | Loud; throughout exhalation | Usually loud; throughout inhalation and exhalation | Absent |
| Pulse/minute | <100 | 100-120 | >120 | Bradycardia |
| Pulsus paradoxus | Absent < 10 mm Hg | May be present 10-25 mm Hg | Often present >25 mm Hg | Absence suggests respiratory muscle fatigue |
| Functional Assessment | ||||
| PEF or FEV1 % predicted or % personal best | ≥70% | 40-69% | <40% | <25% |
| PaO2 (on air, mm Hg) | Normal1 | ≥601 | <60: possible cyanosis | <60: possible cyanosis |
| PCO2 (mm Hg) | <421 | <421 | ≥421 | ≥421 |
| SaO2 (on air) | >95%1 | 90-95%1 | <90%1 | <90%1 |
Adapted from National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. National Institutes of Health Pub. No. 08-4051. Bethesda, MD, 2007.
Figure 9-1. Management of Mild or Moderate Asthma Exacerbations in Primary Care 
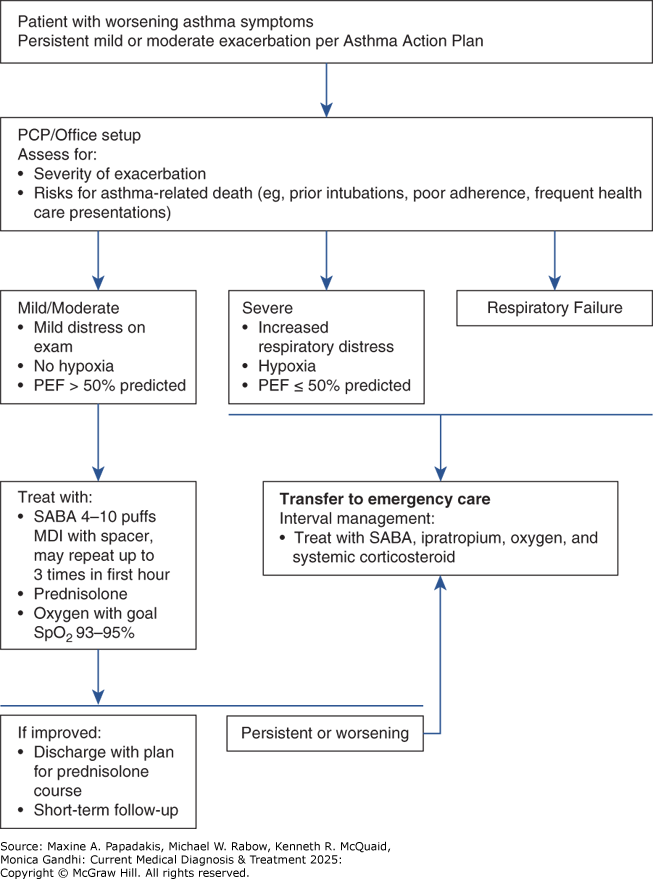
Management of mild or moderate asthma exacerbations in primary care. SABA, short-acting beta-2-agonist (doses are for salbutamol).
A. Mild to Moderate Exacerbations
Mild asthma exacerbations are characterized by only minor changes in airway function (PEF greater than 60% of best) with minimal symptoms and signs of airway dysfunction. Many such patients respond quickly and fully to an inhaled SABA alone, which may need to be continued at increased doses, eg, every 3-4 hours for 24-48 hours. Patients may also require a short-term increase in inhaled corticosteroid to four times the usual dose. In patients not improving after 48 hours, a 5- to 7-day course of oral corticosteroids (eg, prednisone 0.5-1.0 mg/kg/day) may be necessary.
The principal goals for treating moderate asthma exacerbations are correcting hypoxemia, reversing airflow obstruction, and reducing the likelihood of obstruction recurrence. Early intervention may lessen the severity and shorten the duration of an exacerbation. Airflow obstruction is treated with continuous administration of an inhaled SABA and the early administration of systemic corticosteroids. Systemic corticosteroids should be given to patients who have a peak flow less than 70% of baseline or who do not respond to several treatments of SABA. Serial measurements of lung function to quantify the severity of airflow obstruction and its response to treatment are useful. The improvement in FEV1 after 30-60 minutes of treatment correlates significantly with the severity of the asthma exacerbation. Serial measurement of airflow in the emergency department may reduce the rate of hospital admissions for asthma exacerbations. Post-exacerbation care planning is important. All patients, regardless of severity, should be provided with (1) necessary medications and how to use them, (2) instruction in self-assessment, (3) a follow-up appointment, and (4) an action plan for managing recurrence.
B. Severe Exacerbations
Severe exacerbations of asthma can be life-threatening and require immediate treatment. Patients should immediately receive oxygen, high doses of an inhaled SABA, and systemic corticosteroids. A brief history pertinent to the exacerbation can be completed while such treatment is being initiated. More detailed assessments, including laboratory studies, add little early on and should be postponed until after therapy is instituted. Early initiation of oxygen therapy is paramount because asphyxia is a common cause of asthma deaths. Supplemental oxygen should be given to maintain an SaO2 greater than 90% or a PaO2 greater than 60 mm Hg. Oxygen-induced hypoventilation is extremely rare in asthmatic patients, and concern for hypercapnia should never delay correction of hypoxemia.
Frequent high-dose delivery of an inhaled SABA is indicated and usually well tolerated in severe airway obstruction. At least three MDI or nebulizer treatments should be given in the first hour of therapy. Some studies suggest that continuous therapy is more effective than intermittent administration of these agents, but there is no clear consensus as long as similar doses are administered. After the first hour, the frequency of administration varies according to improvements in airflow and symptoms and occurrence of side effects. Ipratropium bromide reduces the rate of hospital admissions when added to inhaled SABAs in patients with moderate to severe asthma exacerbations.
Systemic corticosteroids are administered as detailed above. Intravenous magnesium sulfate (2 g intravenously over 20 minutes) is not recommended for routine use in asthma exacerbations. However, a 2 g infusion over 20 minutes may reduce hospitalization rates in acute severe asthma (FEV1 less than 25% of predicted on presentation or failure to respond to initial treatment).
Mucolytic agents (eg, acetylcysteine, potassium iodide) may worsen cough or airflow obstruction. Anxiolytic and hypnotic drugs are generally contraindicated in severe asthma exacerbations because of their potential respiratory depressant effects.
Multiple studies suggest that infections with viruses (rhinovirus) and bacteria (Mycoplasma pneumoniae, Chlamydophila pneumoniae) predispose to acute exacerbations of asthma and may underlie chronic, severe asthma. The use of empiric antibiotics is, however, not recommended in routine asthma exacerbations since there is no consistent evidence to show improved clinical outcomes. Antibiotics should be considered when there is a high likelihood of acute bacterial respiratory tract infection, such as when patients have fever or purulent sputum and evidence of pneumonia or bacterial sinusitis.
In the emergency department setting, repeat assessment of patients with severe exacerbations should be done after the initial dose of an inhaled SABA and again after three doses of an inhaled SABA (60-90 minutes after initiating treatment). The response to initial treatment is a better predictor of the need for hospitalization than is the severity of the exacerbation on presentation. The decision to hospitalize a patient should be based on the duration and severity of symptoms, severity of airflow obstruction, ABG results (if available), course and severity of prior exacerbations, medication use at the time of the exacerbation, access to medical care and medications, adequacy of social support and home conditions, and presence of psychiatric illness. In general, discharge to home is appropriate if the PEF or FEV1 has returned to 60% or more of predicted or personal best and if symptoms are minimal or absent. Patients with a rapid response to treatment should be observed for 30 minutes after the most recent dose of bronchodilator to ensure stability of response before discharge.
In the intensive care setting, a small subset of patients will not respond to treatment and will progress to impending respiratory failure due to a combination of worsening airflow obstruction and respiratory muscle fatigue (see Figure 9-2 and Table 9-6. Evaluation and Classification of Severity of Asthma Exacerbations). Since such patients can deteriorate rapidly, they must be monitored in a critical care setting. Intubation of an acutely ill asthma patient is technically difficult and is best done semi-electively before the crisis of a respiratory arrest. At the time of intubation, the patient's intravascular volume should be closely monitored because hypotension commonly follows the administration of sedative medications and the initiation of positive-pressure ventilation; these patients are often dehydrated due to poor recent oral intake and high insensible losses.
Figure 9-2. Management of Asthma Exacerbations in Acute Care Facility (Eg, Emergency Department) 
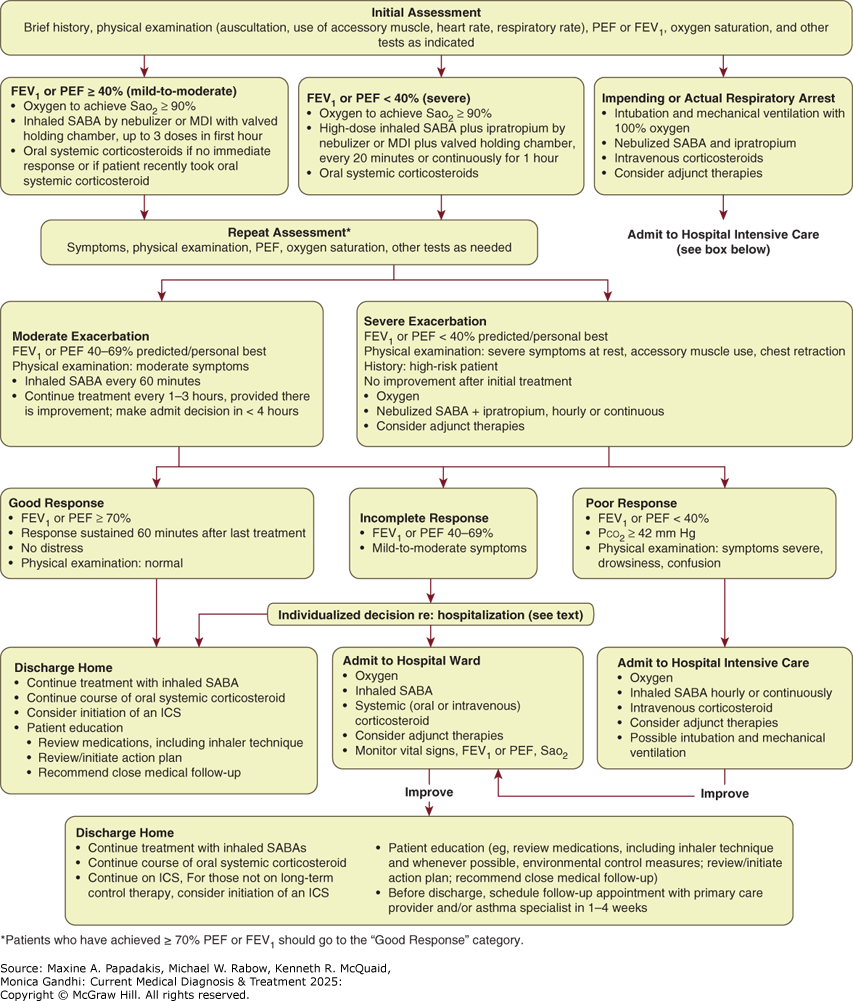
Management of asthma exacerbations in acute care facility (eg, emergency department). FEV1, forced expiratory volume in 1 second; ICS, inhaled corticosteroids; MDI, metered-dose inhaler; PEF, peak expiratory flow; SABA, short-acting beta-2-agonist. (Reproduced with permission from Hasegawa K, Craig SS, Teach SJ, Camargo CA Jr. Management of Asthma Exacerbations in the Emergency Department. J Allergy Clin Immunol Pract. 2021;9(7):2599-2610.)
The main goals of mechanical ventilation are to ensure adequate oxygenation and to avoid barotrauma. Controlled hypoventilation with permissive hypercapnia is often required to limit airway pressures. Frequent high-dose delivery of inhaled SABAs should be continued along with anti-inflammatory agents as discussed above. Many questions remain regarding the optimal delivery of inhaled SABAs to intubated, mechanically ventilated patients. Further studies are needed to determine the comparative efficacy of MDIs and nebulizers, optimal ventilator settings to use during drug delivery, ideal site along the ventilator circuit for introduction of the delivery system, and maximal acceptable drug doses. Unconventional therapies such as helium-oxygen mixtures and inhalational anesthetic agents are of unclear benefit but may be appropriate in selected patients.
When to Refer
- Atypical presentation or uncertain diagnosis of asthma, particularly if additional diagnostic testing is required (bronchoprovocation challenge, allergy skin testing, rhinoscopy, consideration of occupational exposure).
- Complicating comorbid problems, such as rhinosinusitis, multiple environmental allergies, suspected allergic bronchopulmonary aspergillosis.
- Occupational asthma.
- Uncontrolled symptoms despite a moderate-dose inhaled corticosteroid and a LABA.
- Patient not meeting goals of asthma therapy after 3-6 months of treatment.
- Frequent asthma-related health care utilization.
- More than two courses of oral corticosteroid therapy in the past 12 months.
- Any life-threatening asthma exacerbation or exacerbation requiring hospitalization in the past 12 months.
- Presence of social or psychological issues interfering with asthma management.
AgacheIet al. EAACI Biologicals Guidelines—recommendations for severe asthma. Allergy. 2021;76:14. [PMID: 32484954] BleeckerERet al. Systematic literature review of systemic corticosteroid use for asthma management. Am J Respir Crit Care Med. 2020;201:276. [PMID: 31525297] ChippsBEet al. 2020 NAEPP guidelines update and GINA 2021-asthma care differences, overlap, and challenges. J Allergy Clin Immunol Pract. 2022;10:S19. [PMID: 34718214] CloutierMMet al. Managing asthma in adolescents and adults: 2020 Asthma Guideline Update From the National Asthma Education and Prevention Program. JAMA. 2020;324:2301. [PMID: 33270095] CloutierMMet al. 2020 Focused Updates to the Asthma Management Guidelines: A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020;146:1217. [PMID: 33280709] Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2023. http://ginasthma.org/ HardyJet al; PRACTICAL study team. Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet. 2019;394:919. [PMID: 31451207] Menzies-GowAet al. Difficult-to-control asthma management in adults. J Allergy Clin Immunol Pract. 2022;10:378. [PMID: 34954122] PapiAet al. European Respiratory Society short guidelines for the use of as-needed ICS/formoterol in mild Asthma. Eur Respir J. 2023;62:2300047. [PMID: 37678955] van Zyl-SmitRNet al. Once-daily mometasone plus indacaterol versus mometasone or twice-daily fluticasone plus salmeterol in patients with inadequately controlled asthma (PALLADIUM): a randomised, double-blind, triple-dummy, controlled phase 3 study. Lancet Respir Med. 2020;8:987. [PMID: 32653075] VenkatesanPet al. 2023 GINA report for asthma. Lancet Respir Med. 2023;11:589. [[PMID: PMID: 37302397] WenzelSE.Severe adult asthmas: integrating clinical features, biology, and therapeutics to improve outcomes. Am J Respir Crit Care Med. 2021;203:809. [PMID: 33326352] |