Description
- High cardiac output (HCO) is a hyperdynamic circulatory state marked by low mean arterial blood pressure (MAP) and tachycardia:
- Cardiac output (CO) > 8 L/min or cardiac index (CI) > 4 L/min/m2
- Systemic vascular resistance (SVR) <900 dynes × seconds/cm5 or SVR index (SVRI) <1680 dynes × seconds/cm5/m2
Epidemiology
Etiology/Risk Factors
- Physiologic causes: Anxiety, exercise, pregnancy, fever
- Common pathologic causes: ESLD, SIRS/sepsis, hyperthyroidism, obesity
- Less common pathologic causes: Arteriovenous (AV) fistula, anemia (Hg <7 g/dL), carcinoid, beriberi, hereditary hemorrhagic telangiectasia, dermatologic diseases, Kaposi sarcoma, multiple myeloma, Paget's disease.
Physiology/Pathophysiology
- Decreased SVR leading to a low MAP is often the primary circulatory derangement leading to HCO. Two principal causes of low SVR are as follows:
- Vasodilated small and precapillary arterioles
- AV shunting, including iatrogenic AV fistulas
- Compensatory mechanisms are triggered by a low SVR and MAP:
- Chronic activation of the sympathetic nervous system (SNS) causes tachycardia and increases plasma catecholamines, vasomotor tone, myocardial contractility, and CO.
- Activated renin-angiotensin-aldosterone system (RAAS) maintains systemic BP by vasoconstriction and sodium retention.
- Nonosmotic hypersecretion of arginine vasopressin results in retention of free water in order to preserve the circulatory volume.
- Heart failure may occur regardless of the etiology of HCO; it is defined by clinical evidence of heart failure and a CI > 4 L/min/m2 (3) [A]. Chronic SNS activation and an excess of catecholamines cause tachycardia, arrhythmogenesis,
 1 receptor down-regulation, increased myocardial oxygen consumption VO2, and tachycardia-mediated dilated cardiomyopathy.
1 receptor down-regulation, increased myocardial oxygen consumption VO2, and tachycardia-mediated dilated cardiomyopathy. - ESLD: Mesenteric vasodilators (nitric oxide [NO], carbon monoxide, cytokines, etc.) cause splanchnic arterial and venous vasodilatation (1) [A].
- Splanchnic arterial dilatation is so profound that it lowers the overall systemic SVR.
- Splanchnic venous dilatation causes pooling of venous blood and functional hypovolemia.
- A dual circulation exists with a (1) [A]:
- Primary derangement causing a low resistance-high capacitance splanchnic circulation
- Compensatory response causing a high resistance-low capacitance extrasplanchnic circulation
- Cirrhotic cardiomyopathy: Describes systolic dysfunction in the setting of HCO and low SVR, diastolic dysfunction, and supportive criteria (e.g., long QTc and abnormal chronotropy due to
 1 receptor down-regulation). Pharmacologic and physiologic stress can unmask an impaired LVEF and elevated LVEDV (1) [A].
1 receptor down-regulation). Pharmacologic and physiologic stress can unmask an impaired LVEF and elevated LVEDV (1) [A]. - SIRS/sepsis: Adequately fluid-resuscitated patients have a hyperdynamic circulation and low SVR. The extent of SVR reduction appears to be related to mortality (2) [B].
- Mechanisms of low SVR: A blunted rise of intracellular Ca2+ in vascular smooth muscle by activated KATP channels, increased expression of NO synthase, and depletion of vasopressin stores due to sustained baroreflex stimulation (2) [A].
- Septic cardiomyopathy presents as an isolated or combined diastolic and systolic dysfunction. The etiology is unknown but appears to include NO, TNF-
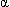 , and cytokines. Impaired LVEF is associated with poor prognosis in septic patients (2) [A].
, and cytokines. Impaired LVEF is associated with poor prognosis in septic patients (2) [A]. - Increased troponins (nonischemic "leak") predict LV dysfunction and mortality (2) [A].
- Hyperthyroidism: Decreased SVR combined with increased chronotropy and inotropy can result in a greater than 250%.