Description
- PaO2 is the partial pressure of oxygen in the arterial blood. Although PaO2 is in equilibrium with dissolved (blood) and bound (oxyhemoglobin) oxygen, these other states do not exert partial pressure.
- Although normal values can be estimated by the use of a simple equation, accurate values must be determined by laboratory arterial blood gas (ABG) analysis.
- Hypoxemia is defined as decreased PaO2 in blood whereas hypoxia is defined as low oxygen availability to the body or to individual organs/tissues
- PAO2 is the alveolar partial pressure of oxygen. In contrast to PaO2, it is a calculated value.
- The partial pressure of a gas is defined as the pressure exerted independently by that gas in a mixture of gases
- According to Dalton's Law, the total pressure of a mixture of gases is equal to the sum of the partial pressures of each individual gas
- The partial pressure of a gas dissolved in a liquid is the partial pressure that would be exerted by the gas while in a gaseous phase and while in equilibrium with the liquid
- According to Henry's Law, if one were to double the pressure of a gas, then the concentration of that gas in solution would be doubled as well
- Solubility is the ability of a substance (O2) to dissolve in a solute (blood). The solubility coefficient of a gas is the volume of the gas that can be dissolved in a certain volume of solvent at a specified temperature and pressure
- At standard body temperature, the solubility coefficient (and the solubility) of CO2 is approximately >20 times that of O2; therefore, diffusion abnormalities should not result in hypercapnia
- The net diffusion of a gas, such as O2, is dependent on the:
- Partial pressure gradient. Since the partial pressure of O2 in the alveoli (PAO2) is greater than the partial pressure of the dissolved O2 in the blood, more O2 molecules will diffuse from the alveoli into the pulmonary capillary blood. It is also dependent on
- Diffusion coefficient. Based on the solubility and molecular weight of the gas in question
- Thickness and surface area of the pulmonary capillary membrane. Because of its tremendous size and microscopic thickness (0.4–0.5 µm), a normal pulmonary capillary membrane provides almost no resistance to the diffusion of lipid soluble gases such as O2 and CO2
- Transit time. The rate limiting step in the transport of O2 from the alveoli to the blood is typically the loading of O2 onto Hb, not the diffusion of O2 across the pulmonary capillary membrane
- Partial pressure gradient. Since the partial pressure of O2 in the alveoli (PAO2) is greater than the partial pressure of the dissolved O2 in the blood, more O2 molecules will diffuse from the alveoli into the pulmonary capillary blood. It is also dependent on
- Diffusion capacity is the amount of gas (O2) that can be transferred from the alveoli to the blood across the alveolar-capillary membrane. The O2 diffusing capacity at rest in an adult male is ~21 mL/min/mm Hg and may increase to 65 mL/min/mm Hg with strenuous exercise
- PaO2 will be <Pc’O2 (pulmonary end-capillary O2) because it will be diluted with mixed venous blood called the venous admixture. Normally, the venous admixture consists of blood from the bronchial-pulmonary venous communication, the Thebesian circulation, and low V/Q areas in the lung
- Hemoglobin is a molecule consisting of 4 heme and 4 protein subunits. Heme is an iron-porphyrin compound comprising an essential part of O2 binding sites; only the divalent (+2 charge) form of iron can bind O2. Hemoglobin A1 (normal adult hemoglobin) consists of 2
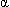 and 2
and 2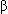 chains held together by weak bonds between the amino acid residues. Theoretically, each gram of Hb can carry up to 1.39 mL of O2.
chains held together by weak bonds between the amino acid residues. Theoretically, each gram of Hb can carry up to 1.39 mL of O2. - Peripheral chemoreceptors include the aortic and carotid bodies; PaO2 has a minimal effect on central chemoreceptors
- The aortic bodies (found in the aortic arch and its branches) have predominantly circulatory effects, while the carotid bodies (located at the bifurcation of the common carotid artery) have predominantly ventilatory effects
- Neural output from the aortic bodies travels to the medullary centers via the vagus (CN X) nerve, while output from the carotid bodies reaches the central respiratory centers via the afferent glossopharyngeal (CN IX) nerve
- Both carotid and aortic bodies are stimulated by decreased PaO2, but not by decreased SaO2 or CaO2
- Neural activity from these receptors begins to increase when the PaO2 falls to <100 mm Hg. When PaO2 levels reach 60–65 mm Hg, neural activity is substantially augmented to increase the minute ventilation
- Patients that depend on hypoxic ventilatory drive (those with COPD, for example) have PaO2 values in the mid 60s; this must be considered when weaning these patients from mechanical ventilatory support as spontaneous ventilation will not resume until the PaO2 falls to <65 mm Hg
Physiology/Pathophysiology
- The oxygen–hemoglobin (also known as the oxyhemoglobin) dissociation curve is the graphical illustration of the measured relationship between PaO2 and SaO2.
- SaO2 is a measure of the percentage of hemoglobin binding sites occupied by O2. A normal PaO2 of 100 mm Hg results in a SaO2 of about 98%
- The curve is sigmoidal in shape; it represents conformational changes to Hb as more O2 molecules bind to, or dissociate from, it. These changes accelerate the loading, or unloading, of the fourth O2 molecule.
- The "S" shape also minimizes the effects of changes in PaO2 on the SaO2, while in the normal range. However, there is a "steep" vertical section where small changes in PaO2 result in significant drops in the SaO2; it also corresponds to the PaO2 level where chemoreceptors begin firing.
- P50 (the point at which Hb is 50% saturated) normally falls at a PaO2 of 26.7 mm Hg.
- If the oxyhemoglobin curve is shifted to the left (reduced P50), then hemoglobin has a higher than normal affinity for O2, resulting in decreased O2 unloading. Circumstances causing a leftward shift include: Abnormal hemoglobin (fetal), alkalosis, carboxyhemoglobin, decreased 2,3-DPG, hypothermia, and methemoglobin. When the curve is shifted to the right (increased P50), Hb has a lower affinity for O2 leading to increased O2 unloading in the periphery. Shifting of the curve to the right may be caused by: Abnormal hemoglobin, acidosis, hyperthermia, increased 2,3-DPG, and increased CO2
- The most common mechanism for hypoxemia is an increased alveolar-arterial gradient
- The diffusion of O2 can be slowed when the thickness of the pulmonary capillary membrane becomes pathologically thickened. This may occur in diseases such as pulmonary fibrosis, pulmonary edema (accumulation of interstitial fluid in the membrane), and pneumonia (inflammatory fluid accumulation in the membrane)
- Dissolved oxygen usually has very little impact on the total arterial oxygen content because most of the oxygen in blood is carried by hemoglobin (see equation )
- Pulse oximetry is listed an American Society of Anesthesiologists Standards for Basic Anesthetic Monitoring. It is a safe, noninvasive, continuous method to measure oxygenation and rapidly detect hypoxia. Oximetry readings provide an estimation of the PaO2, based on the oxygen–hemoglobin curve.
- Values are based on the Lambert–Beer law stating that oxyhemoglobin and deoxyhemoglobin absorb different quantities of red and infrared light
- Oxyhemoglobin (HbO2) absorbs more infrared light at a wavelength of 940 nm and therefore, appears red to the human eye
- Deoxyhemoglobin (Hb) absorbs more red light at 660 nm, appearing blue or cyanotic
- Oxygen saturation is calculated by a microprocessor that compares the ratio of light absorption at the red and infrared wavelengths
- Functional oximetry is defined as HbO2 divided by the sum of HbO2 and Hb; it does not include COHb and MetHb which, in normal physiological states, are present in small concentrations. The operating room and ICU pulse oximeter are functional pulse oximeters.
- Fractional oximetry is defined as the ratio of HbO2 to the total Hb (all 4 species: HbO2, Hb, COHb, MetHb). It is measured by laboratory co-oximeters.
- The pulse oximeter isolates the pulsatile component of blood based on plethysmography, in an attempt to eliminate false readings from venous blood or tissue
- Pulse oximeters also measure heart rate based on this pulsatile flow in addition to the SpO2
- Although generally very accurate at SpO2 readings from 70 to 100%, pulse oximeters have several potential problems
- Values reflect an average value over a time span of 5–8 seconds; this delay can translate into a delay in treatment.
- Artifacts may be caused by fingernail polish, ambient light, methylene blue dye, and motion.
- Low perfusion states such as cardiac arrest, hypothermia, increased SVR, severe anemia, cardiac bypass, or tourniquet placement may affect the ability to obtain a reading
- Carboxyhemoglobin (COHb) absorbs light in a similar manner to HbO2, resulting in a falsely high reading in patients with CO poisoning
- Methemoglobin absorbs light equally at red and infrared wavelengths, providing a constant pulse oximeter reading of ~85%. SpO2 may be falsely high or low depending on the actual SaO2
- Unable to distinguish between PaO2 values greater than 100 mmHg – will all have oxygen saturations of 100%
- ABG analysis measures PaO2 values as well as arterial pH, PaCO2, HCO3-, and base deficit. It allows for an assessment of oxygenation (PaO2), ventilation based on the PaCO2, and provides insight into the acid–base status with pH measurement and base deficit.
- Normal range of PaO2 in an adult is 60–100 mm Hg and is dependent on multiple factors including alveolar ventilation, FiO2 (inspired O2 concentration), SvO2 (mixed venous O2 saturation), and ventilation–perfusion ratio
- If PaO2 is <60 mm Hg, then hypoxemia is present
- If hypoxemia is present, calculation of the A-a gradient will help to determine the cause
- Normal A-a gradient may result from hypoventilation or low FiO2
- Increased A-a gradient can be caused by V/Q mismatching, increased right to left shunting, and diffusion abnormalities
- Approximation of arterial oxygen tension based on age = PaO2 = 102 – Age/3
- Alveolar gas equation = PAO2 = FiO2 (PB – PH2O) – PACO2/R; PACO2/R is used an as indirect measure of VO2/VA where VO2 is O2 consumption and VA is alveolar ventilation
- Arterial Oxygen Content = CaO2 = O2 bound to Hb + O2 dissolved in blood = [SaO2 × (Hb × 1.34)] + [PaO2 × 0.0031]
- Oxygen Delivery = DO2 = CO × CaO2
- Dalton's Law = Ptotal = Pgas1 + Pgas2 + PgasN
- Henry's Law = Cg = k × Pg, where Cg is concentration of the gas in solution, k is a solubility constant, and Pg is the partial pressure of the gas
- Diffusion Capacity for O2 = DLO2 = Oxygen Uptake/(PAO2 – PC’O2)
- Functional Oximetry = SpO2 = HbO2/(HbO2 + Hb)
- Fractional Oximetry = SaO2 = HbO2/(HbO2 + Hb + COHb + MetHb + other hemoglobins)