Solutions vary in terms of their components and the concentration of those components. The two major categories of infusion therapy solutions are differentiated by the size of the particles that make them up: crystalloid (small particles that form crystals) and colloid (large particles that do not break down in water). This chapter focuses on crystalloid solutions because they are routinely administered intravenous solutions. Two major components of crystalloid solutions are dextrose (D) and sodium chloride (NaCl). Sodium chloride solutions are also called saline solutions and are available in various concentrations, distinguished by fractions or percentages (Table 10-1). Normal saline (NS) is 0.9% sodium chloride and is termed "normal" because its osmolarity, or particles per liter, is approximately the same as that of plasma. Dextrose solutions are available in 5%, 10%, 20%, and 50% concentrations, denoted by a number, often written as a subscript, in the solution name—for example, D5W is the abbreviated name for a solution of 5% dextrose (D5) in water (W). Dextrose 5% is typically combined with saline, as shown in Table 10-1. Another common crystalloid, lactated Ringer's solution (LR), is a combination of fluid and electrolytes.
Commonly Prescribed Intravenous Solutions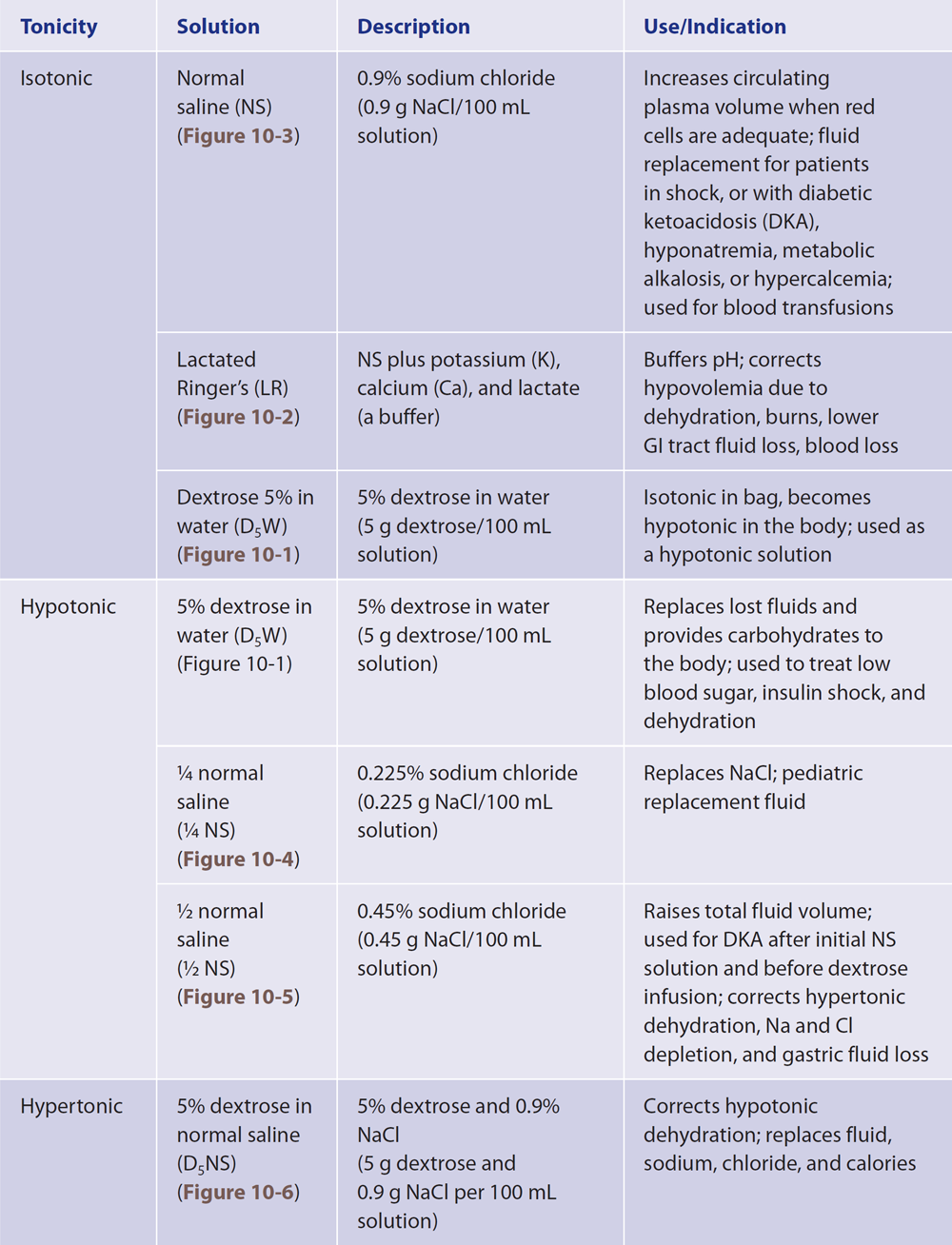
A table is titled Commonly Prescribed Intravenous Solutions.
The table consists of four columns: Tonicity, Solution, Description, and Use and Indication. Row entries are as follows. Row 1. Tonicity: Isotonic. Solution: Normal saline, N S, Figure 10-3. Description: 0.9 percent sodium chloride, 0.9 grams N a C l per 100 milliliters solution. Use and Indication: Increases circulating plasma volume when red cells are adequate; fluid replacement for patients in shock, or with diabetic ketoacidosis, D K A, hyponatremia, metabolic alkalosis, or hypercalcemia; used for blood transfusions. Row 2. Tonicity: Isotonic. Solution: Lactated Ringer's, L R, Figure 10-2. Description: N S plus potassium, K, calcium, C a, and lactate, a buffer. Use and Indication: Buffers p H; corrects hypovolemia due to dehydration, burns, lower G I tract fluid loss, blood loss. Row 3. Tonicity: Isotonic. Solution: Dextrose 5 percent in water, D subscript 5 W, Figure 10-1. Description: 5 percent dextrose in water, 5 grams dextrose per 100 milliliters solution. Use and Indication: Isotonic in bag, becomes hypotonic in the body; used as a hypotonic solution. Row 4. Tonicity: Hypotonic. Solution: 5 percent dextrose in water, D subscript 5 W, Figure 10-1. Description: 5 percent dextrose in water, 5 grams dextrose per 100 milliliters solution. Use and Indication: Replaces lost fluids and provides carbohydrates to the body; used to treat low blood sugar, insulin shock, and dehydration. Row 5. Tonicity: Hypotonic. Solution: 1 over 4 normal saline, 1 over 4 N S, Figure 10-4. Description: 0.225 percent sodium chloride, 0.225 grams N a C l per 100 milliliters solution. Use and Indication: Replaces N a C l; pediatric replacement fluid. Row 6. Tonicity: Hypotonic. Solution: 1 over 2 normal saline, 1 over 2 N S, Figure 10-5. Description: 0.45 percent sodium chloride, 0.45 grams N a C l per 100 milliliters solution. Use and Indication: Raises total fluid volume; used for D K A after initial N S solution and before dextrose infusion; corrects hypertonic dehydration, N a and C l depletion, and gastric fluid loss. Row 7. Tonicity: Hypertonic. Solution: 5 percent dextrose in normal saline, D subscript 5 N S, Figure 10-6. Description: 5 percent dextrose and 0.9 percent N a C l, 5 grams dextrose and 0.9 grams N a C l per 100 milliliters solution. Use and Indication: Corrects hypotonic dehydration; replaces fluid, sodium, chloride, and calories. The table consists of four columns: Tonicity, Solution, Description, and Use and Indication. Row entries are as follows. Row 8. Tonicity: Hypertonic. Solution: 5 percent dextrose in lactated Ringer's solution, D subscript 5 L R, Figure 10-7. Description: 5 percent dextrose in lactated Ringer's solution, 5 grams dextrose per 100 milliliters lactated Ringer's solution. Use and Indication: Provides water, electrolytes, and calories; used for hypovolemic forward slash hemorrhagic shock; used as an alkalinizing agent to correct acidosis. Row 9. Tonicity: Hypertonic. Solution: 5 percent dextrose in 0.225 percent N a C l, 5 grams dextrose and 0.225 grams N a C l per 100 milliliters solution. Use and Indication: Provides free water for insensible fluid loss; promotes renal function and excretion. Used postoperatively with added potassium, as a maintenance fluid. Row 10. Tonicity: Hypertonic. Solution: 5 percent dextrose in 1 over 2 normal saline, D subscript 5 1 over 2 N S, Figure 10-9. Description: 5 percent dextrose in 0.45 percent N a C l, 5 grams dextrose and 0.45 grams N a C l per 100 milliliters solution. Use and Indication: Used for D K A after initial treatment with N S and 21 over 2 N S solution; prevents hypoglycemia and cerebral edema, occurs when serum osmolality is reduced rapidly.
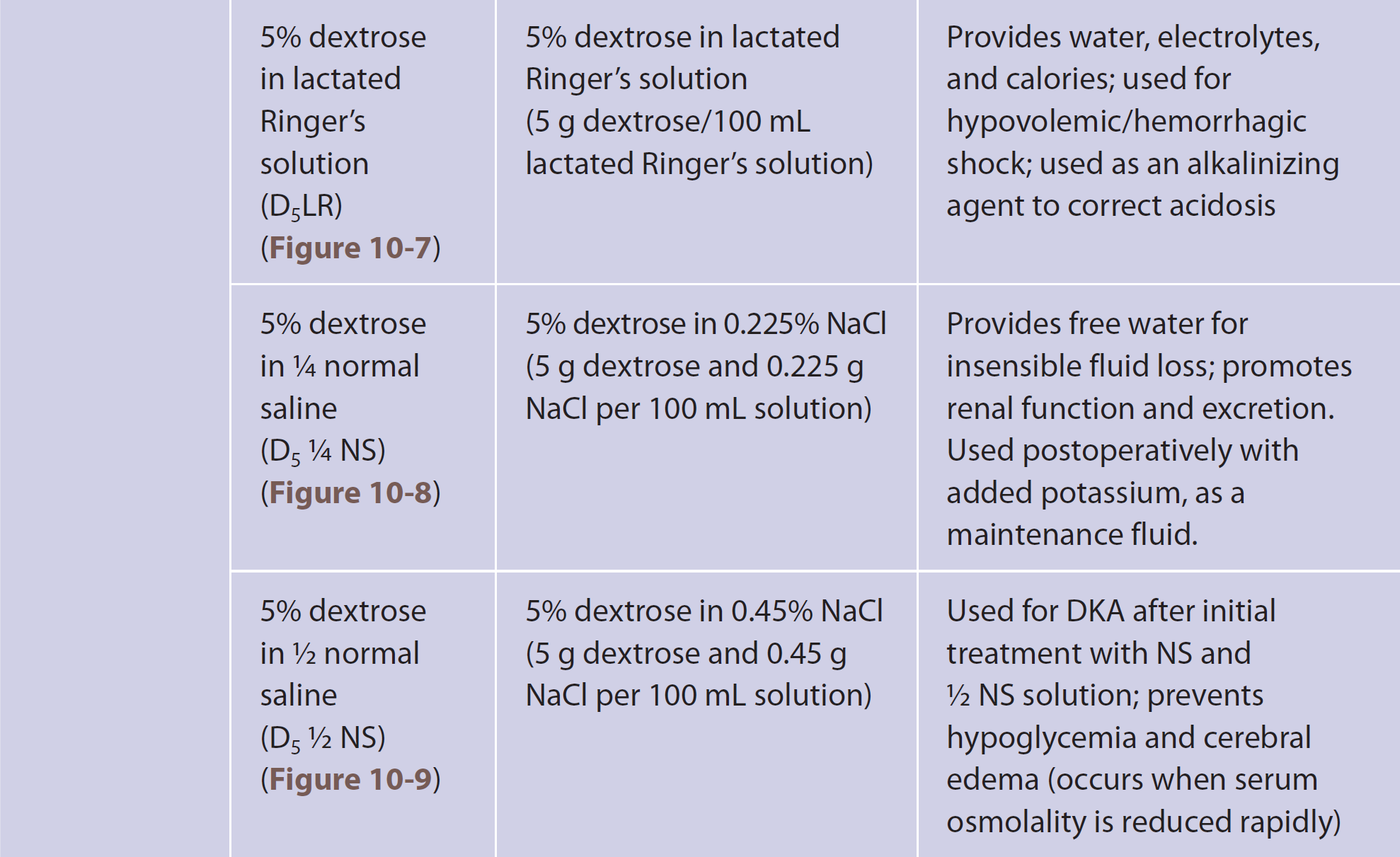
A table is titled Commonly Prescribed Intravenous Solutions, continued.
Lippincott Nursing Center. (2022, December). Intravenous (IV) fluids. Retrieved from https://www.nursingcenter.com/getattachment/Clinical-Resources/nursing-pocket-cards/IV-Fluids/Pocket-Card_IV-Fluids_December2022.pdf.aspx Vera, M. 2023, February 12. Intravenous fluids and solutions guide cheat sheet. Nurselabs. Retrieved from https://nurseslabs.com/iv-fluidsolution-quick-reference-guide-cheat-sheet/.
Dextrose 5% in water, D5W, has an initial osmolarity similar to that of intravascular fluid (isotonic), but this concentration of dextrose is rapidly metabolized, changing the osmolality of the solution (to hypotonic).
An illustration of a bag containing 5 percent dextrose U S P for injection.
Text in the bag reads, 250 milliliters. Each 100 milliliter contains Dextrose, hydrous 5 grams in water for injection, or 252 milliosmoles per liter, calculated. p H 4.3, 3.2 to 6.5. Dextrose solutions without salts should not be used in blood transfusions because of possible Rouleau formation. Do not use in series connection. For intravenous use only. Use only if solution is clear and container and seals are intact. Warnings: Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store. Sterile, nonpyrogenic. Single-dose container. Store at room temperature at 25 degrees Celsius. Avoid excessive heat. Protect from freezing. See package insert. R x only. Latex-free, P V C free, D E H P free. Do not remove overwrap until ready for use. After removing the overwrap, check for minute leaks by squeezing container firmly. If leaks are found, discard solution as sterility may be impaired.
Abbreviated LR and also known as Ringer's lactate (RL) or Hartmann's solution, lactated Ringer's solution is the most physiologically adaptable fluid because its electrolyte content is most closely related to the composition of the body's serum and plasma.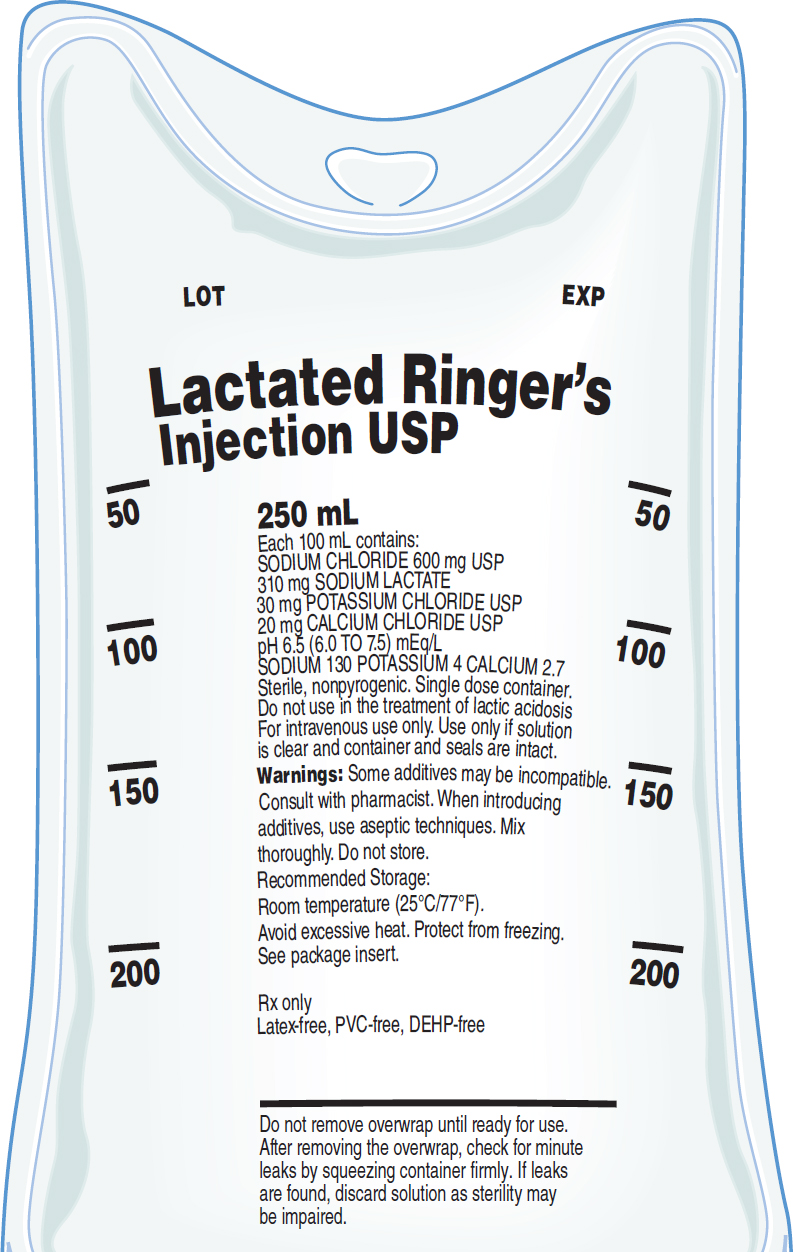
An illustration of a bag containing lactated ringer's U S P for injection.
Text in the bag reads, 250 milliliters. Each 100 milliliter contains Sodium chloride 600 milligrams, U S P. 310 milligrams sodium lactate. 30 milligrams potassium chloride U S P. 20 milligrams calcium chloride U S P. p H 6.5, 6.0 to 7.5, milliequivalents per liter. Sodium, 130, potassium, 4, calcium, 2.7. Sterile, nonpyrogenic. Single-dose container. Do not use in the treatment of lactic acidosis For intravenous use only. Use only if solution is clear and container and seals are intact. Warnings: Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store. Recommended Storage: Room temperature at 25 degrees Celsius or 77 degrees Fahrenheit. Avoid excessive heat. Protect from freezing. See package insert. R x only. Latex-free, P V C free, D E H P free. Do not remove overwrap until ready for use. After removing the overwrap, check for minute leaks by squeezing container firmly. If leaks are found, discard solution as sterility may be impaired.
Normal saline (NS) is 0.9% sodium chloride solution.
An illustration of a bag containing 0.9 percent sodium chloride U S P for injection.
Text in the bag reads, 250 milliliters. Each 100 milliliter contains Sodium chloride 900 milligrams, p H 5.6 or 4.5 to 7.0. The solution is isotonic, 308 millimoles per liter, calculated. Electrolyte content in milliequivalents per liter, N a cations 154, C l anions,154, Sterile, nonpyrogenic. Single-dose container. Do not use in series connection. For intravenous use only. Use only if solution is clear and container and seals are intact. Warnings: Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store. Recommended Storage: Room temperature 25 degrees Celsius. Avoid excessive heat. Protect from freezing. See package insert. R x only. Latex-free, P V C-free, D E H P-free. Do not remove overwrap until ready for use. After removing the overwrap, check for minute leaks by squeezing container firmly. If leaks are found, discard solution as sterility may be impaired.
Because 0.225% is one-fourth of 0.9%, 0.225% sodium chloride solution is called ¼ NS.
An illustration of a bag containing 0.225 percent sodium chloride U S P for injection.
Text in the bag reads, 250 milliliters. Each 100 milliliter contains Sodium chloride, 225 milligrams. p H 4.3, 3.5 to 6.5. The solution is isotonic, 329 milliosmoles per liter, calculated. Electrolyte content in milliequivalents per liter, Na cations 38.5, C l anions, 38.5. Sterile, nonpyrogenic. Single-dose container. Do not use in series connection. For intravenous use only. Use only if solution is clear and container and seals are intact. Warnings: Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store. Recommended Storage: Room temperature 25 degrees Celsius. Avoid excessive heat. Protect from freezing. See package insert. R x only. Latex-free, P V C free, D E H P free. Do not remove overwrap until ready for use. After removing the overwrap, check for minute leaks by squeezing container firmly. If leaks are found, discard solution as sterility may be impaired.
Because 0.45% sodium chloride is half of 0.9%, 0.45% sodium chloride solution is called ½ NS.
An illustration of a bag containing 0.45 percent sodium chloride U S P for injection.
Text in the bag reads, 250 milliliters. Each 100 milliliter contains Sodium chloride U S P, 0.45 grams. Water for injection U S P qs. pH 5.6, 4.5 to 7.0. p H adjusted with H C l N F 155 milliosmoles per liter, calculated. Hypotonic. Electrolyte content in milliequivalents per liter, N a cations 77, C l anions, 77. Sterile, nonpyrogenic. Single-dose container. Do not use in series connection. For intravenous use only. Use only if solution is clear and container and seals are intact. Warnings: Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store. Recommended Storage: Room temperature 25 degrees Celsius. Avoid excessive heat. Protect from freezing. See package insert. R x only. Latex-free, P V C free, D E H P free. Do not remove overwrap until ready for use. After removing the overwrap, check for minute leaks by squeezing container firmly. If leaks are found, discard solution as sterility may be impaired.
Dextrose 5% and 0.9% sodium chloride is called D5NS.
An illustration of a bag containing 5 percent dextrose and 0.9 percent sodium chloride U S P for injection.
Text in the bag reads, 500 milliliters. Each 100 milliliter contains Dextrose, hydrous, 5 grams. Sodium chloride, 0.9 milligrams in water for injection. p H 4.3, 3.5 to 6.5. 329 milliosmoles per liter, calculated. Electrolyte content in milliequivalents per liter, N a cations 38.5, C l anions, 38.5. Sterile, nonpyrogenic. Single-dose container. Do not use in series connection. For intravenous use only. Use only if solution is clear and container and seals are intact. Warnings: Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store. Recommended Storage: Room temperature 25 degrees Celsius. Avoid excessive heat. Protect from freezing. See package insert. R x only. Latex-free, P V C free, D E H P free. Do not remove overwrap until ready for use. After removing the overwrap, check for minute leaks by squeezing container firmly. If leaks are found, discard solution as sterility may be impaired.
Dextrose 5% in lactated Ringer's solution, D5LR, is a replacement fluid and can be used to correct acidosis (except lactic acidosis) because lactate, when metabolized, converts to bicarbonate, thereby acting as a buffer (acid neutralizer).
An illustration of a bag containing 5 percent dextrose in lactated ringer's U S P for injection.
Text in the bag reads, 250 milliliters. Each 100 milliliter contains Hydrous dextrose, 5 grams. Sodium chloride 0.6 grams, U S P. 0.31 grams sodium lactate. 0.03 grams potassium chloride U S P. 0.02 grams calcium chloride U S P. p H 4.6, 4.0 to 6.0, milliequivalents per liter. Sodium, 130, potassium, 4, calcium, 2.7. Sterile, nonpyrogenic. Single-dose container. Do not use in the treatment of lactic acidosis For intravenous use only. Use only if solution is clear and container and seals are intact. Warnings: Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store. Recommended Storage: Room temperature at 25 degrees Celsius or 77 degrees Fahrenheit. Avoid excessive heat. Protect from freezing. See package insert. R x only. Latex-free, P V C free, D E H P free. Do not remove overwrap until ready for use. After removing the overwrap, check for minute leaks by squeezing container firmly. If leaks are found, discard solution as sterility may be impaired.
Dextrose 5% and 0.225% sodium chloride solution is called D5 ¼ NS.
An illustration of a bag containing 5 percent dextrose and 0.225 percent sodium chloride U S P for injection.
Text in the bag reads, 500 milliliters. Each 100-milliliter contains Dextrose, hydrous, 5 grams. Sodium chloride, 225 milligrams in water for injection. pH 4.3, 3.5 to 6.5. 329 milliosmoles per liter, calculated. Electrolyte content in milliequivalents per liter, Na cations 38.5, C l anions, 38.5. Sterile, nonpyrogenic. Single dose container. Do not use in series connection. For intravenous use only. Use only if solution is clear and container and seals are intact. Warnings: Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store. Recommended Storage: Room temperature 25 degrees Celsius. Avoid excessive heat. Protect from freezing. See package insert. R x only. Latex-free, P V C free, D E H P free. Do not remove overwrap until ready for use. After removing the overwrap, check for minute leaks by squeezing container firmly. If leaks are found, discard solution as sterility may be impaired.
The addition of dextrose 5% provides calories and free water to the hypotonic solution of 0.45% sodium chloride, making D5 ½ NS a good choice for replacement fluids.
An illustration of a bag containing 5 percent dextrose and 0.45 percent sodium chloride U S P for injection.
Text in the bag reads, 500 milliliters. Each 100 milliliter contains Dextrose, hydrous U S P, 5 grams. Sodium chloride U S P, 0.45 grams. Water for injection U S P qs. p H 4.4, 3.5 to 6.5. 405 milliosmoles per liter, calculated. Hypotonic. Electrolyte content in milliequivalents per liter, Na cations 77, Cl anions, 77. Sterile, nonpyrogenic. Single-dose container. Do not use in series connection. For intravenous use only. Use only if solution is clear and container and seals are intact. Warnings: Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store. Recommended Storage: Room temperature 25 degrees Celsius. Avoid excessive heat. Protect from freezing. See package insert. R x only. Latex-free, P V C free, D E H P free. Do not remove overwrap until ready for use. After removing the overwrap, check for minute leaks by squeezing container firmly. If leaks are found, discard solution as sterility may be impaired.