Population: Patients at risk for ASCVD considering statin therapy.
Organizations
 USPSTF 2022, ACC/AHA 2018, 2019, ESC/EAS 2019, CCS 2021, VA-DoD 2020
USPSTF 2022, ACC/AHA 2018, 2019, ESC/EAS 2019, CCS 2021, VA-DoD 2020
Prevention Recommendation
–A variety of guidelines exist to guide thresholds to initiate statin therapy.

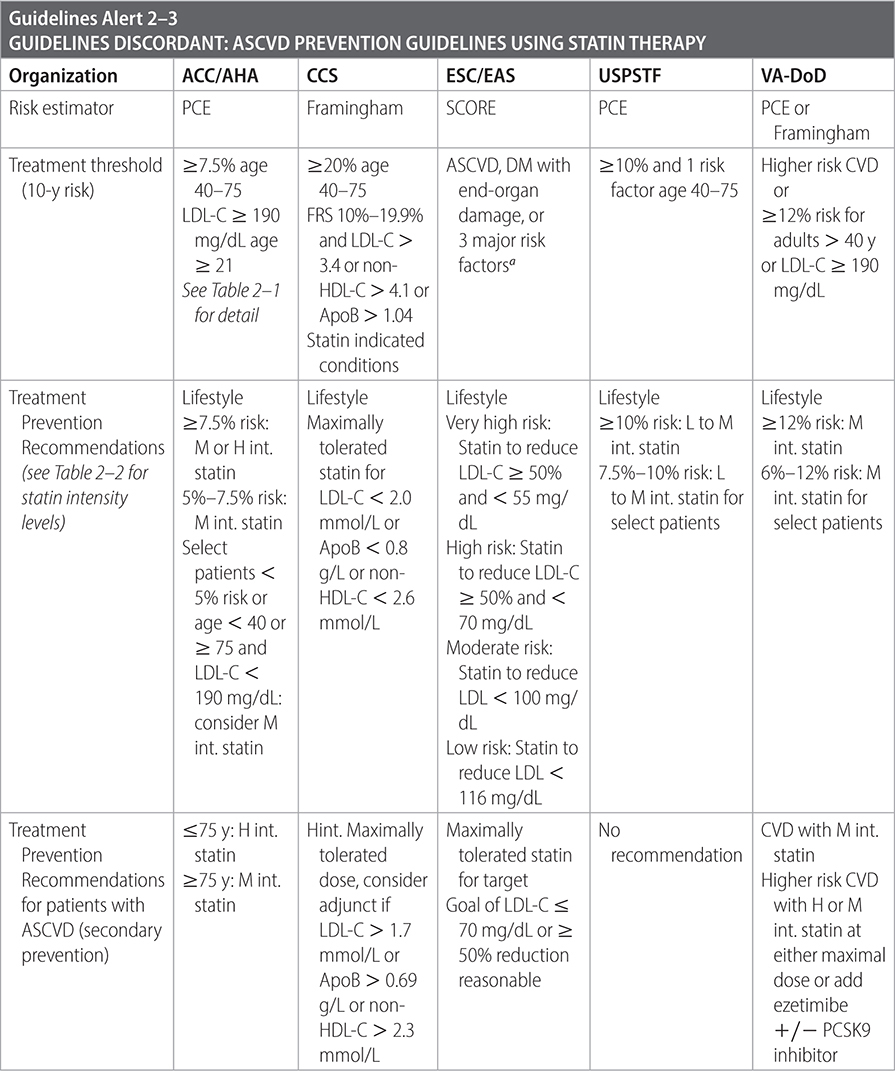

Sources
–USPSTF. JAMA. 2022;328(8):746-753.
–ACC/AHA. J Am Coll Cardiol. 2019;73(24):e285-e350.
–Eur Heart J. 2020;41(1):111-188.
–Canadian J Cardiol. 2021;37(1129-1150).
–VA/DoD Clinical Practice Guidelines: The Management of Dyslipidemia for Cardiovascular Risk Reduction. 2020.
TABLE 2–1 ASCVD GROUPS THAT BENEFIT FROM STATIN THERAPY
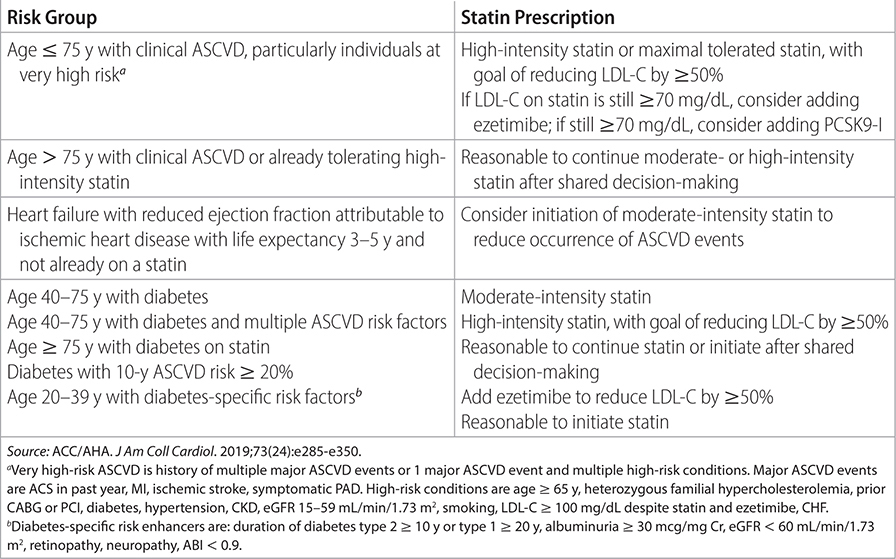
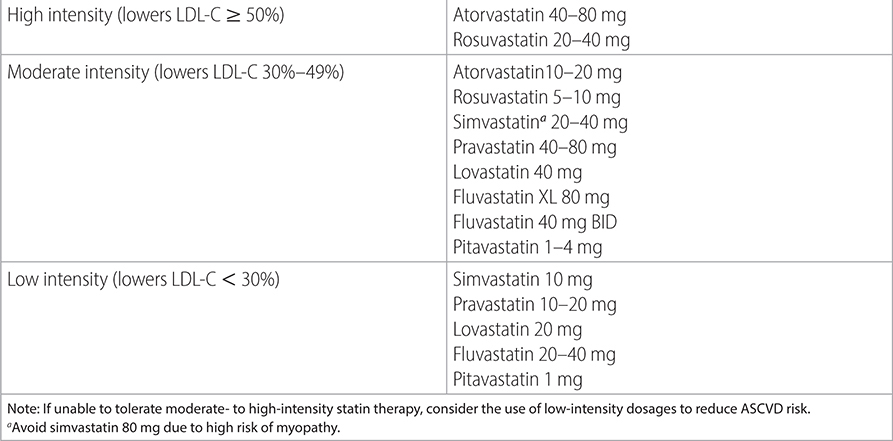
TABLE 2–2 STATIN INTENSITY DRUG LEVELS
Practice Pearls
• ACC/AHA: If risk is uncertain, assess coronary artery calcium. Potential candidates for coronary artery calcium measurement include:
- Patients reluctant to start statin therapy, who want to understand risk/benefits.
- Patients who may want to know the benefits of statin therapy after discontinuation due to side effects.
- Older men age 55–80 or women age 60–80 with fewer risk factors, who question whether they would benefit from statin therapy.
- Adults age 40–55 in borderline risk group (with ASCVD risk 5%–7.5%) with other factors that increase their risk.
• Recommend lifestyle management and drug therapy for high-risk groups.
• In patients intolerant of statin, consider reducing dose or switching to an alternate agent unless reaction is severe. See Table 2–3 for listing of proven adverse effects of statins.
• Consider combination of statin and non-statin therapy (refer to Table 2–4) in select patients.
• CCS:
- For Primary Prevention:
• Consider add-on therapy with ezetimibe as first-line if LDL-C > 1.9 mmol/L or ApoB > 0.7 g/L or non-HDL-C > 2.6 mmol/L on maximally tolerated statin.
- For Secondary Prevention:
• Recommends adjunct treatment to maximally tolerated statin be PCSK9 inhibitor (in patients with highest benefit or LDL-C > 2.2 mmol/L or ApoB > 0.8 g/L or non-HDL-C > 2.9 mmol/L) +/− ezetimibe.
• Consider icosapent ethyl 2000 mg BID if TG is >2.4 to <5.6 mmol/L in patients already receiving maximally tolerated statin.
- Statin Indicated Conditions:
• LDL > 4.9 mmol/L.
• Most patients with DM: >39 y or >29 y with >14 y duration or microvascular disease.
• CKD: >49 y and eGFR < 60 mL/min/1.73m2.
• VA/DoD:
- For higher risk groups, shared decision-making should be used regarding harms and benefits when deciding between moderate- or high-intensity statins.
- For patients needing intensified therapy: Recommends maximal dosing of statin or ezetimibe prior to PCSK9 inhibitors due to safety profile and efficacy.
- Recommends against using niacin or omega-3 fatty acids and against adding fibrates to statins, for primary or secondary prevention.
- Consider icosapent ethyl in patients on statin therapy with persistent fasting TG > 150 mg/dL for secondary prevention only.
Sources
–ACC/AHA. J Am Coll Cardiol. 2019;73(24):e285-e350.
–ACC/AHA. J Am Coll Cardiol. 2019;74(10):e177-e232.
TABLE 2–3 STATIN-ASSOCIATED SIDE EFFECTS
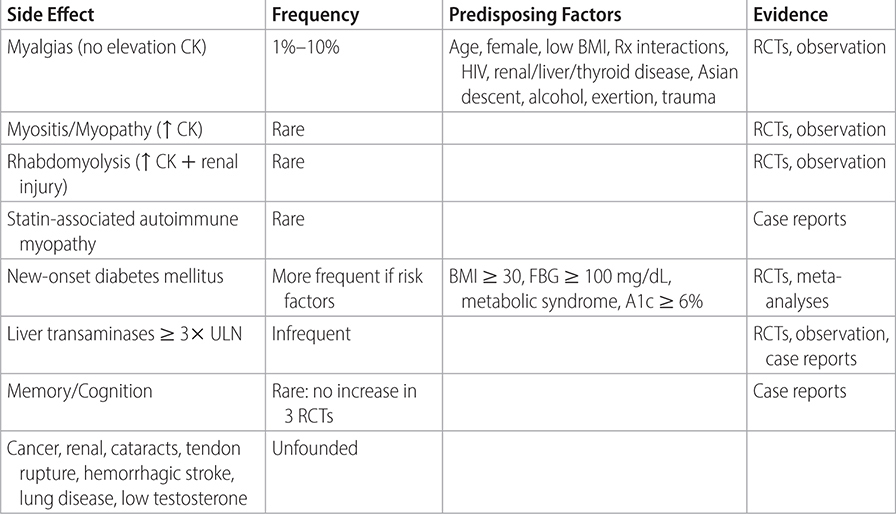
TABLE 2–4 NON-STATIN CHOLESTEROL-LOWERING AGENTS |
|---|
• In high-risk patients (clinical ASCVD, age < 75 y; LDL-C ≥ 190 mg/dL; 40- to 75-y-old with DM) who are intolerant to statins, the use of non-statin cholesterol-lowering drugs may be considered. • Niacin: Indicated for LDL-C elevation or fasting triglyceride ≥ 500 mg/dL; avoid with liver disease, persistent hyperglycemia, acute gout, or new-onset AF. • BAS: Indicated for LDL-C elevation; avoid with triglycerides ≥ 300 mg/dL. • Ezetimibe: Indicated for LDL-C elevation; when combined with statin, monitor transaminase levels. • Fibrates: Indicated for fasting triglycerides ≥ 500 mg/dL. If needed, consider adding fenofibrate only to a low- or moderate-intensity statin. Avoid the addition of gemfibrozil to statin agent due to increased risk of muscle symptoms. Avoid fenofibrate if moderate/severe renal impairment. • Omega-3 fatty acids: Indicated in severe fasting triglycerides ≥ 500 mg/dL. • PCSK9 (proprotein convertase subtilisin kexin 9) inhibitors: FDA-approved monoclonal antibodies including alirocumab (Praluent®) and evolocumab (Repatha®). Studies have shown decrease in LDL cholesterol most notably in patients with heterozygous familial hypercholesterolemia. FOURIER trial tested evolocumab in combination with statin therapy against placebo plus statin therapy in patients with elevated cholesterol levels and existing CVD. There was a modest additional reduction in LDL and composite cardiovascular events. (N Engl J Med. 2017;376:1713-1722) |
Source: Adapted from J Am Coll Cardiol. 2018;71:794-799. |