Population: Nonpregnant adults with type 2 DM.
Management of adults with comorbid coronary artery disease and hypertension is discussed in Chapter 19: Cardiovascular Diseases.
Management of adults with comorbid coronary kidney disease is discussed in Chapter 31: Renal Disorders.
Management of pregnant people with diabetes is discussed in the section “Diabetes Mellitus, Gestational” of Chapter 29.
Organizations
 AACE/ACE 2020, ACP 2017/2018, ADA 2023, ESC 2019, NICE 2022, Endocrine Society 2022, KDIGO 2022, ACSM 2022
AACE/ACE 2020, ACP 2017/2018, ADA 2023, ESC 2019, NICE 2022, Endocrine Society 2022, KDIGO 2022, ACSM 2022
Recommendations
Management: Lifestyle Interventions
–Weight loss: Recommend >5% weight loss if overweight or obese; consider adjunctive weight loss medications approved by FDA. (ADA, AACE, IDF) Consider more intensive weight loss goals (ie, 15%) to maximize benefit depending on need, feasibility, and safety. Larger, sustained weight losses (>10%) usually confer greater benefits, including disease-modifying effects and possible remission of T2D, and may improve long-term cardiovascular outcomes and mortality. (ADA)
–Exercise: Regular physical activity (150 min/wk moderate aerobic activity), supplemented with 2–3 resistance, flexibility, and/or balance training sessions/wk, behavioral intervention. (ADA, AACE, IDF, ACSM) Encourage reduced sedentary time and breaking up sitting time with frequent small “doses” of activity throughout the day. (ADA, ACSM)
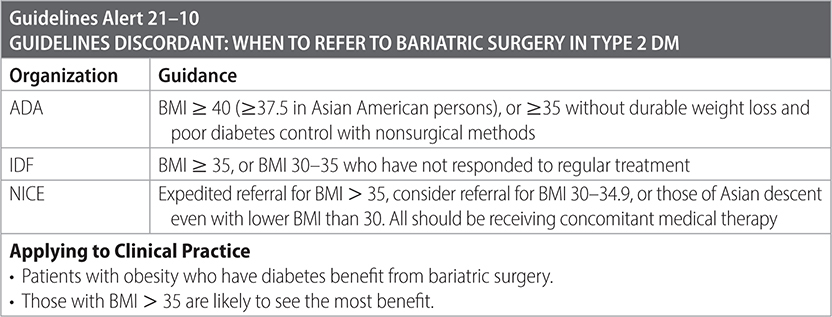
–Recommend bariatric surgery for good surgical candidates who meet criteria.
–Team-based care: Use a multidisciplinary model (including physicians, nurses, dietitians, exercise specialists, dentists, podiatrists, mental health, etc.) to assess barriers to care, including food, housing, and financial insecurity as well as literacy and numeracy. Refer to available community resources. Consider socioeconomic factors that may impact nutrition choices, such as food insecurity and cultural circumstances. (ADA)
–Provide diabetes self-management education and support at appropriate intervals including education about hypoglycemia management and adjustments during illness. Complement in-person visits with telehealth and other digital solutions to optimize glycemic management and offer self-management education and clinical support. (ADA, Endocrine Society)
–Advise against tobacco use, including e-cigarettes (given evidence of associated deaths), and provide smoking cessation counseling/treatment routinely.
Management: Medications
–Individualize medication regimens and blood glucose targets (fasting and postprandial) based on patient-specific factors (likely adherence, safety, efficacy, cost, and comorbidities such as cardiac, cerebrovascular, hepatic, and renal disease). (AACE, NICE, ADA)
–Use metformin as first-line medication.1
–Start with metformin monotherapy unless A1c is >9%. (ADA)
–Combination therapy is usually necessary. Choose agents with complementary mechanisms of action, especially in patients with comorbidities. See Table 21–1 for a more detailed description of various agents available, and Tables 21–2 and 21–3 for further guidance for patients with specific comorbidities.
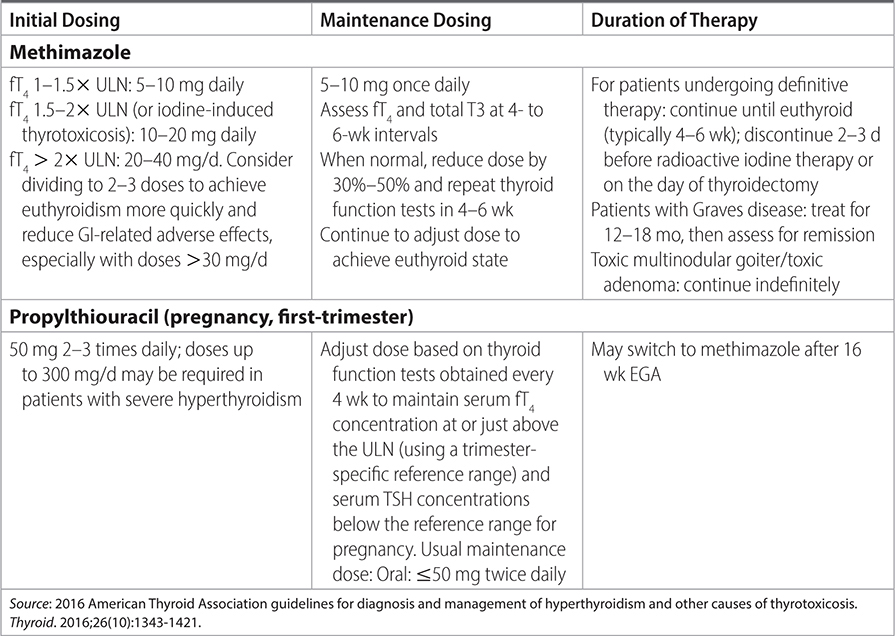
TABLE 21–1 DOSING AND MONITORING OF ANTITHYROID DRUG THERAPY
–Add either a sulfonylurea, a thiazolidinedione (pioglitazone), an SGLT2 inhibitor, or a DPP-4 inhibitor to metformin when a second agent is required, based on discussion of benefits, adverse effects, and cost. (ACP, NICE)
–Consider starting two agents if the HbA1c is 1%–2% or more above target. Combination therapy should be metformin plus sulfonylurea (SU),1 DPP4 inhibitor, SGLT2 inhibitor,2 or GLP-1 receptor agonist.3 (IDF)
–When selecting a regimen, consider approaches that support weight management goals. Dual GLP-1/glucose-dependent insulinotropic polypeptide (GIP) receptor agonists are a glucose-lowering option with the potential for weight loss. (ADA)
TABLE 21–2 RECOMMENDATIONS FOR ADJUNCTIVE DIABETES THERAPIES COMPLEMENTARY TO COMMON COMORBIDITIES
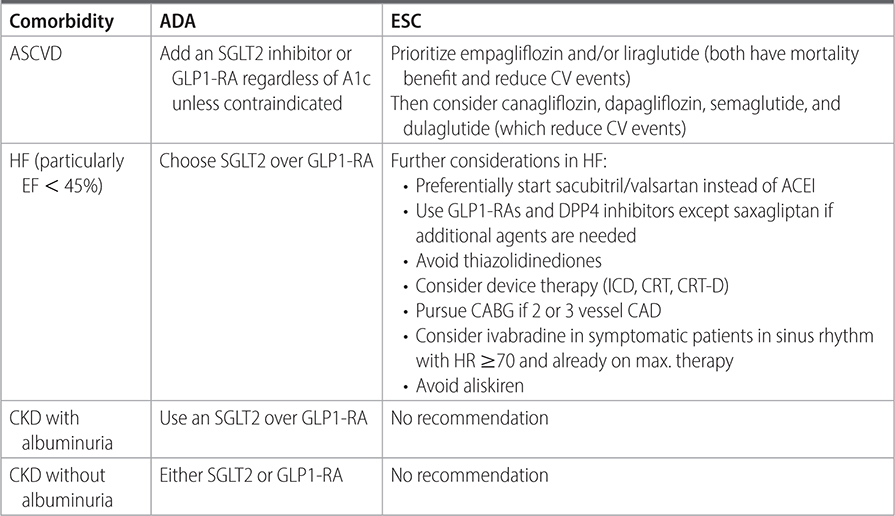
TABLE 21–3 INTERNATIONAL PANEL GUIDELINE ON SELECTING SGLT-2 INHIBITORS OR GLP-1 RECEPTOR AGONISTS

–Consider combination therapy with metformin and GLP-1 RA or SGL2i for patients with renal or cardiovascular comorbidities. Use of GLP-1-RA or SGLT2i may be appropriate as monotherapy if there is a contraindication to metformin. (ADA, NICE)
–Use SGLT2i for patients with DM2 and comorbidities including CVD, HFpEF/HFrEF, and CKD with eGFR ≥ 20 mL/min/1.73 m2 and UACR > 3.0 mg/mmol (>30 mg/g) independent of use of metformin or baseline HbA1C in patients with these comorbidities. (ADA)
–In patients with DM2 and comorbid CVD, HF, CKD and contraindications or intolerance to SGLT2-i use, consider a GLP-1 RA to reduce cardiovascular events until renal replacement therapy is indicated. (ADA)
Management: Insulin
–Consider initiating insulin in the following situations: ongoing catabolic weight loss, symptomatic hyperglycemia, A1c > 10% or BG > 300 mg/dL. (ADA)
–Consider starting insulin after addition of second or third oral agent and HbA1c > 7%. (NICE)
• Initial insulin therapy: NPH once or twice daily versus long-acting insulin.
• Consider basal bolus versus premixed biphasic regimen if A1c > 9%.
–See Fig. 21–1 for a consolidated insulin initiation strategy.
–When titrating insulin, be cautious of overbasalization (basal dose > 0.5 U/kg, high bedtime–morning glucose differential, or hypoglycemia) and adjust therapy as appropriate. (ADA)
–Prefer GLP-1-RA when combination therapy is needed, as it may address prandial control while minimizing risks of hypoglycemia and weight gain associated with insulin therapy. (ADA)
FIG. 21–1 STRATEGIES FOR INSULIN INITIATION AND TITRATION IN TYPE 2 DM.
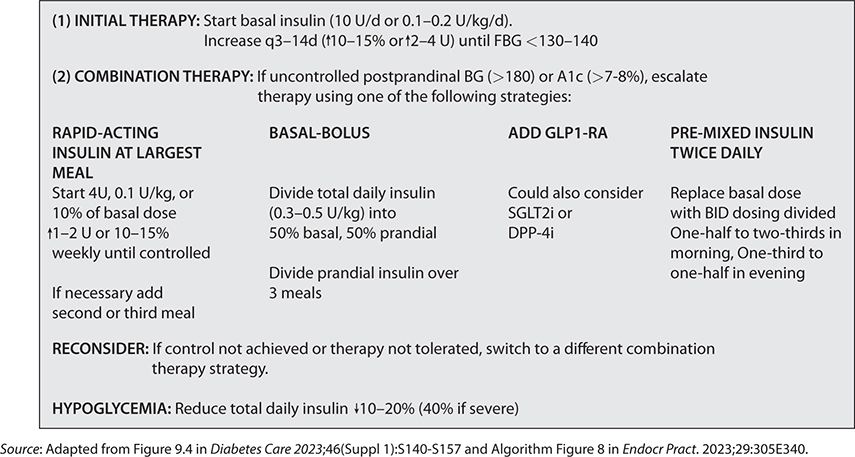
–If insulin is used, choose combination therapy with a GLP-1 RA1 as it has greater efficacy, more durable treatment effect, and more weight and hypoglycemia benefit. (ADA)
–Glycemic control recommendations [ADA]:
• Pre-prandial glucose: 80–130 mg/dL.
• Postprandial glucose: <180 mg/dL (1–2 h post meals).
• CGM parameters: TIR (“time-in-range”) should be used to assess glucose control (associated with risk of microvascular complications) and “time below/above target” should be used to reevaluate treatment regimen.
Preventing Complications
–Minimize the risks of hypoglycemia, weight gain, and other adverse drug reactions. (AACE)
–Hypoglycemia: Assess for symptomatic/asymptomatic hypoglycemia (BG < 70 mg/dL) at each visit. Prescribe IM or intranasal glucagon to patients at high risk of level 2 hypoglycemia (BG < 54 mg/dL). Reevaluate treatment regimen and glycemic targets if hypoglycemia unawareness or level 3 hypoglycemia (altered mental/physical functioning requiring assistance). (ADA)
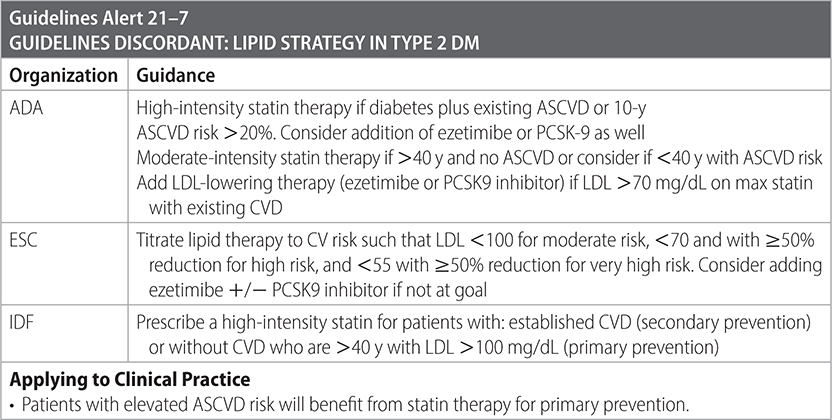
–Control lipids and BP. (AACE)
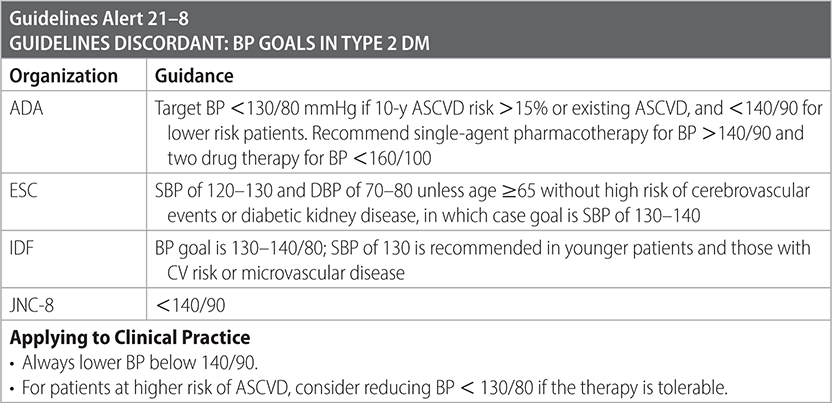
–Optimize BP alongside glycemic control to prevent progression of diabetic kidney disease.
–Instruct patients with T2DM and HTN to monitor their BP at home. (ADA)
–Recommend ACEI or ARB for patients with hypertension and diabetes with urinary albumin-to-creatinine ratio >30 mg/g creatinine or eGFR < 60 mL/min/1.73 m2. (ADA, IDF)
• Start ACEI/ARB if patient has microalbuminuria without high BP. (IDF)
–Additional therapy with CCB or thiazide diuretic as needed to reach BP goals. (ADA)
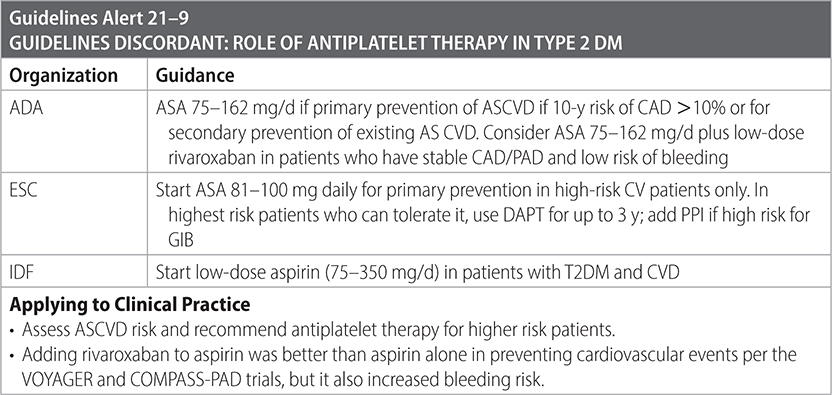
–Antiplatelet agents for primary and secondary prevention of CVD are indicated.
–Maintain updated vaccination status (ADA) with pneumococcal, influenza, hepatitis B, HPV, Tdap, Zoster, and COVID-19 vaccines. See “Immunization Schedule” in Ch 34 for details.
–Refer patients with any evidence of diabetic retinopathy to ophthalmologist. (ADA, NICE)
–Patients who observe Ramadan should interrupt their fast if SMBG is <70 or >300. High-risk patients are advised not to fast at all.
–Advise on higher risk of periodontitis and recommend regular oral health reviews, as managing periodontitis can improve glycemic control. (NICE)
Surveillance
–Monitor therapy q 3 mo until stable, then at least twice a year. (AACE, ADA)
–On follow-up, address interval medical history, medication adherence, side effects including hypoglycemia, lab evaluation, nutrition, psychosocial health, routine health maintenance screening. (AACE, ADA)
–Perform foot examination at every visit for high-risk patients, and annually for lower risk patients. (ADA, IDF, NICE)
–Screen for or check annually:
• Lipid panel if on statin therapy (otherwise, q 5 y is sufficient). (ADA)
• Dilated fundoscopic exam or retinal photography q 1–2 y. (ADA, IDF)
• Monofilament screening for diabetic neuropathy. (ADA, IDF)
• Screen for peripheral vascular disease by checking foot pulses and/or calculating the ankle/brachial index. (ADA)
• Depression with PHQ-2. (IDF)
 Consider screening for sleep health, including sleep disorders and sleep disruptions due to diabetes symptoms or management needs. Refer to sleep medicine and/or behavioral health professional as indicated. (ADA)
Consider screening for sleep health, including sleep disorders and sleep disruptions due to diabetes symptoms or management needs. Refer to sleep medicine and/or behavioral health professional as indicated. (ADA)




Practice Pearls
• Metformin may cause vitamin B12 deficiency; consider periodic monitoring of vitamin B12 levels especially in patients with anemia or neuropathy. Avoid if eGFR < 30 mL/min/1.73 m2 or unstable HF.
• Consider GLP-1 receptor agonist as first-line injectable medication before insulin.
• ACEIs or ARBs are first-line antihypertensives. Second-line antihypertensives are dihydropyridine calcium channel blockers, thiazide diuretic if GFR ≥ 30 mL/min/1.73 m2 or a loop diuretic if GFR < 30 mL/min/1.73 m2.
• Metformin, smoking cessation, BP control, and statins consistently improve cardiovascular outcomes.
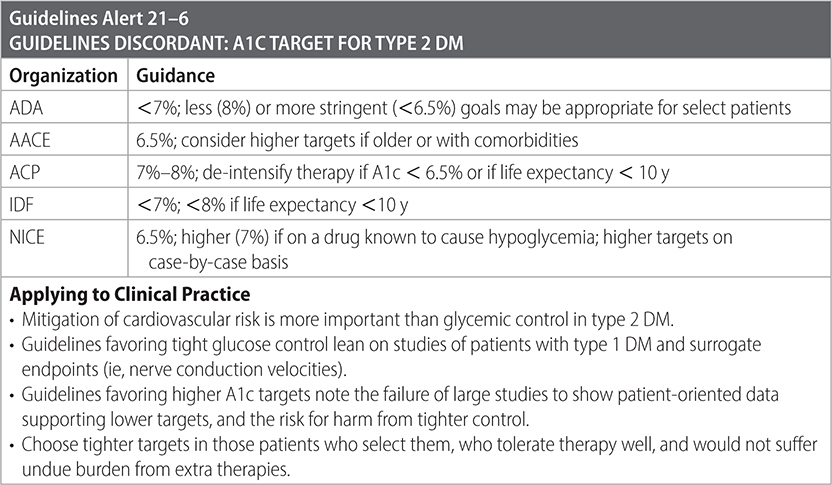
• Glycemic control is a staple of care, but controversy exists regarding optimal A1c goals for ambulatory adults. Advocates of tighter control (ie, <7) rely on data from several trials (ACCORD, ADVANCE, UKPDS, VADT) that show improvements in surrogate markers (ie, nerve conduction velocity) but not patient-oriented outcomes (ie, painful neuropathy or mortality) with tighter control. The DCCT trial showed 50%–76% reductions in development and progression of microvascular complications. Long-term follow-up demonstrated persistence of microvascular benefit over two decades, independent of persistence of glycemic control. Advocates of more permissive control (ie, <8%) point to potential harm from medication burden and hypoglycemia and the absence of evidence for patient-oriented benefit in tighter control of type 2 DM. (ADA)
• The largest trials for glycemic control (such as listed above) do not include SGLT2 inhibitors or GLP-1 receptor agonists, which may provide some cardiovascular benefit apart from glycemic reduction.
• Continuous glucose monitoring is now recommended for anyone on multiple daily insulin injections, regardless of type of diabetes or age.
• Self-monitoring of blood glucose levels in patients not on insulin is controversial. There are no agreed upon frequencies within the guidelines (once/d vs. twice/d vs. twice/wk). It is reasonable to consider self-monitoring when making medication changes, diet changes, or alterations in physical activity. It is also reasonable to prescribe self-monitoring in patients on medications with known side-effects of hypoglycemia (such as sulfonylureas).
• The ODYSSEY OUTCOMES trial demonstrated statistically significant absolute risk reduction in primary endpoints (death from CAD, non-fatal MI, non-fatal ischemic stroke, unstable angina) in patients with T2DM with recent ACS who were treated with combination statin and Alirocumab (PCSK-9). Consider dual therapy in post-ACS patients with T2DM.
• In patients with ASCVD risk factors with elevated triglycerides (135–499), but controlled LDL cholesterol on a statin, consider treatment with icosapent ethyl.
• Though guidelines suggest NPH and regular insulins are less efficacious and carry increased risk of hypoglycemia, the hypoglycemia risk is small and these formulations are typically less costly than preferred therapy. They may be appropriate for selected patients. (Endocrine Society, ADA).
Sources
–https://pro.aace.com/pdfs/diabetes/algorithm-exec-summary.pdf
–https://academic.oup.com/eurheartj/article/41/2/255/5556890
–https://diabetesjournals.org/care/issue/46/Supplement_1
–Ann Intern Med. 2017;166:279-290.
–Ann Intern Med. 2018;168:569-576.
–International Diabetes Federation. Recommendations for Managing Type 2 Diabetes in Primary Care. 2017. www.idf.org/managing-type2-diabetes
–https://www.nice.org.uk/guidance/ng28
–Endocrine Society. Management of Individuals with Diabetes at High Risk for Hypoglycemia: An Endocrine Society Clinical Practice Guideline. 2022. https://doi.org/10.1210/clinem/dgac596
–KDIGO Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. 2022.
–ACSM. Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine. 2022. doi:10.1249/MSS.0000000000002800
–Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753-2786. https://doi.org/10.2337/dci22-0034
Population: Hospitalized adults with type 2 DM.
Organizations
 AAFP 2017, ADA 2023, Endocrine Society 2022
AAFP 2017, ADA 2023, Endocrine Society 2022
Recommendations
–Avoid intensive insulin therapy in hospitalized patients (even in ICU).
–Target blood glucose level of 140–180 mg/dL if insulin therapy is used in hospitalized patients, especially those who are critically ill. More stringent goals, such as 110–140 mg/dL (6.1–7.8 mmol/L) or 100–180 mg/dL (5.6–10.0 mmol/L), may be appropriate for selected patients and are acceptable if they can be achieved without significant hypoglycemia. (ADA)
–Use either basal insulin or basal plus bolus correctional insulin in the treatment of hospitalized patients; sliding scale regimens alone are no longer recommended.
–For patients receiving glucocorticoid therapy, consider ordering NPH simultaneously with their steroid to prevent worsening daytime and prandial hyperglycemia. (ADA)
–Support continued use of devices such as insulin pumps and CGM systems during hospitalization if competency is established and proper management/supervision is available. (ADA, Endocrine Society)
Practice Pearl
• Intensive insulin therapy in SICU/MICU patients does not improve mortality but has a 10- to 15-fold increased risk of hypoglycemia.
Sources
–https://www.aafp.org/journalpdfrestricted/afp/2017/1115/p648.pdf
–https://diabetesjournals.org/care/issue/46/Supplement_1
–Endocrine Society. Management of Individuals with Diabetes at High Risk for Hypoglycemia: An Endocrine Society Clinical Practice Guideline. 2022. https://doi.org/10.1210/clinem/dgac596
Population: Children and adolescents with newly diagnosed DM2.
Organizations
 AAP 2021, ADA 2023, NICE 2022
AAP 2021, ADA 2023, NICE 2022
Recommendations
Management
–Recommend diet, exercise, and lifestyle modification for all those diagnosed.
–Offer Diabetes Self-Management Education and Support (DSMES). (ADA)
–Limit nonacademic screen time to <2 h/d.
–Assess psychological and social situation.
–Advise all patients not to smoke.
–Monitor HbA1c every 3 mo.
–Use metformin as first-line therapy.
–Add liraglutide if insufficient control on metformin alone.
–Start insulin therapy if:
• DKA.
• A1c > 8.5% on metformin + liraglutide.
• Random glucose > 250 mg/dL.

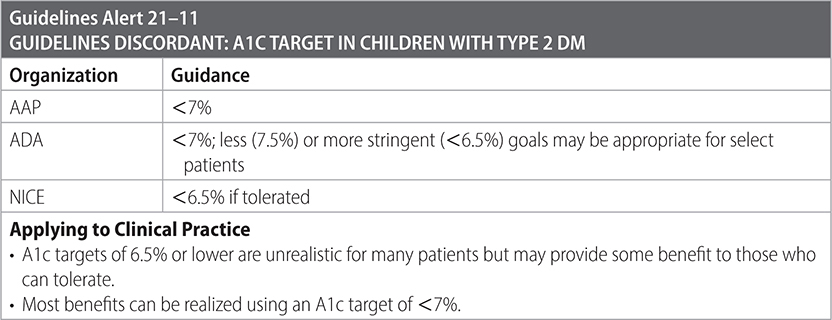
Surveillance
–BP measurement at every clinical visit.
• If BP ≥ 95th percentile despite lifestyle management, start ACEI/ARB. (ADA)
–Screening for PCOS at time of diagnosis for adolescent females. (ADA)
–If LDL persistently ≥ 130 after 6 mo of medical nutrition therapy, start statin for goal LDL < 100. (ADA)
–If triglycerides are >400 fasting or >1000 nonfasting, treat with fibrate. (ADA)
–Screen annually for:
• Albuminuria: Urine albumin-to-creatinine ratio.
• Dyslipidemia: Lipid panel.
• Neuropathy: Comprehensive foot examination and monofilament testing.
• Retinopathy: Retinal exam.
• NAFLD: LFTs. (ADA)
• Symptoms of OSA. (ADA)
Sources
–pediatrics.aappublications.org/content/131/2/364.full.pdf