Cyclophosphamide and Ifosfamide.
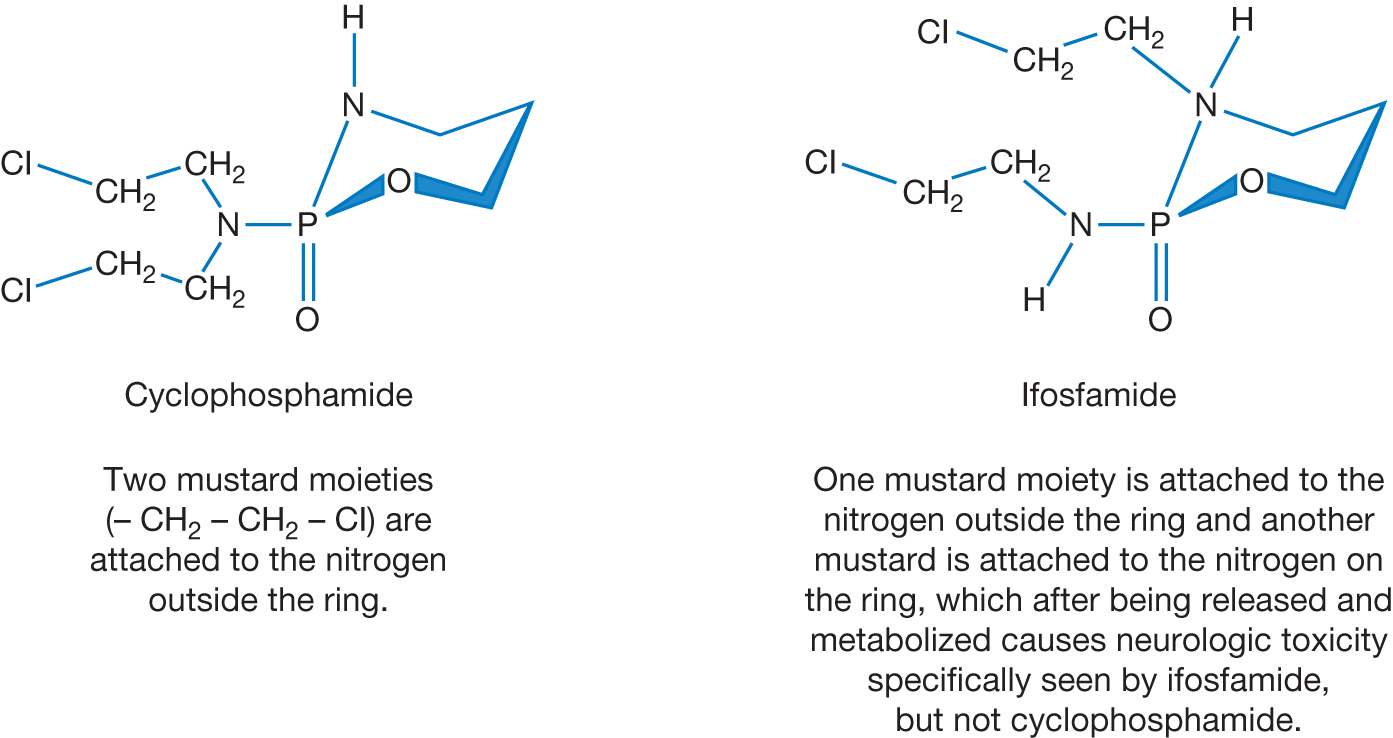
Cyclophosphamide and ifosfamide are prodrugs. After intravenous or oral administration, cyclophosphamide, in the liver via cytochrome P450, is converted to 4-hydroxycyclophosphamide, which stays in equilibrium with aldophosphamide, both of which readily cross the cell membranes by passive diffusion. In cells with high levels of aldehyde dehydrogenase (ALDH; e.g., hematopoietic stem cells), aldophosphamide is irreversibly converted to carboxyphosphamide, which does not decompose to phosphoramide mustard and therefore lacks alkylating activity. In the absence of a high concentration of ALDH (e.g., lymphocytes), aldophosphamide spontaneously liberates phosphoramide mustard and acrolein. Phosphoramide mustard forms interstrand DNA crosslinks primarily at the guanine sites (Figure 2.1). The differences between ALDH expression in hematopoietic stem cells and lymphocyte has resulted in the wide use of high-dose cyclophosphamide after blood and marrow transplantation to prevent/diminish graft versus host disease (GVHD). Alloreactive T cells, but not stem cells, which are activated a few days after allogeneic transplantation, become exquisitely susceptible to killing by high-dose cyclophosphamide. Owing to its stem cell sparing effects, high-dose cyclophosphamide (50 mg/kg on days 3 and 4 after transplant) has been found to be an effective method for clinical GVHD prophylaxis. Cyclophosphamide is used for treatment of many lymphoid malignancies as well as solid tumors such as breast cancer.
Metabolism of ifosfamide defers from cyclophosphamide, resulting in a particular adverse event profile. The first step of metabolism of ifosfamide involves the release of “ring nitrogen mustard” to chloroacetaldehyde (CAA). CAA can penetrate the blood–brain barrier resulting in significant central nervous system (CNS) toxicity manifested by cerebellar ataxia, mental confusion, and complex visual hallucinations. This adverse reaction can be abrogated by administration of methylene blue, which acts as a potent electron acceptor and inhibits the extrahepatic monoamine oxidation of chloroethylamine to CAA. Due to the lack of ring nitrogen mustard, cyclophosphamide does not cause CNS toxicity similar to ifosfamide. Ifosfamide is used for treatment of solid tumors, including testicular cancer, soft tissue sarcoma, and osteosarcoma.