[
Show Section Outline]
DESCRIPTION
N-acetylcysteine (NAC), an antidote for acetaminophen poisoning, was originally used as an inhalation mucolytic agent.
FORMS AND USES
- Pharmaceutical preparations include Mucomyst and Acetylcysteine Solution USP.
- NAC is available as a 10% or 20% solution for inhalation or oral administration.
MECHANISM OF ACTION
Several mechanisms of action have been proposed:
- NAC acts as a precursor of glutathione, which detoxifies the toxic metabolite of acetaminophen, N-acetyl-p-benzoquinoneimine (NAPQI).
- Providing NAC as a substrate for glutathione synthesis may prevent depletion of glutathione stores associated with acetaminophen toxicity and thereby prevent liver injury.
- The sulfhydryl group of NAC may bind and detoxify NAPQI directly.
- Donation of a sulfur group by NAC may drive the metabolism of acetaminophen toward the nontoxic sulfation pathway and away from its toxic pathway.
- NAC may improve hepatic microcirculation and reduce acetaminophen toxicity.
- NAC may be a hepatic antioxidant, attenuating oxidant-induced acetaminophen liver injury.
PREGNANCY AND LACTATION
- US FDA Pregnancy Category B. Animal studies indicate no fetal risk, and there are no controlled human studies, or animal studies show an adverse fetal effect but well-controlled studies in pregnant women do not.
- Pregnant women with toxic serum acetaminophen levels as determined by the Rumack-Matthew nomogram should receive a full course of NAC.
- An infant delivered to a woman receiving NAC for acetaminophen toxicity should receive a full course of NAC, typically by the intravenous route.
Section Outline:
[
Show Section Outline]
ACUTE SINGLE INGESTION
- The decision to treat with NAC is determined by plotting the patient's serum or plasma acetaminophen level on the Rumack-Matthew acetaminophen nomogram (see figure below).
- The acetaminophen level must be drawn between 4 and 24 hours after ingestion. If the level plots above the "possible toxicity" line, the patient should be treated with NAC.
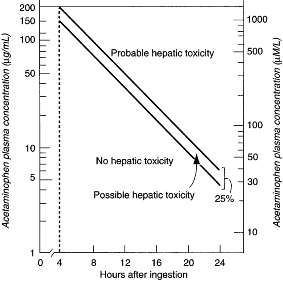
Semilogarithmic plot of plasma acetaminophen levels versus time. (Modified and reproduced, with permission, from Rumack BH, Matthew H. Acetaminophen poisoning and toxicity. Pediatrics 1975;55:871.)
ACETAMINOPHEN, ATYPICAL OVERDOSE CONDITIONS
- Known acute overdose, but the time of ingestion is uncertain or unknown.
- Possible acute overdose where elevated results on liver function tests are present; increase in levels of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) indicate acetaminophen injury until proven otherwise.
- Known acute overdose patient that presents more than 48 hours after ingestion. NAC is not needed if liver enzymes are normal, but patient should receive NAC if liver enzymes are abnormal.
- Patients with a history of a single acute overdose between 24 and 36 hours prior to presentation should receive NAC therapy regardless of whether measurable acetaminophen is present or whether liver function tests are normal, because liver toxicity may not become apparent until 24 to 48 hours postingestion.
- Repeated ingestion of acetaminophen rather than single ingestion. If ALT or AST are abnormal, the patient should be treated with NAC. If ALT and AST are normal and acetaminophen level is undetectable, the patient does not need NAC therapy.
OTHER POISONINGS TREATED WITH NAC
- Chlorinated hydrocarbon poisoning (carbon tetrachloride, chloroform). NAC is the proposed therapy, but efficacy has not been studied.
- Amanita phalloides mushroom poisoning. NAC is the proposed therapy, but efficacy is unproven.
- Monochloroacetic acid. NAC is the proposed therapy, but efficacy is unproven.
- Cyclophosphamide. Severity of cyclophosphamide-induced hemorrhagic cystitis is reduced by NAC.
- Doxorubicin. Possible prevention by NAC of doxorubicin-induced cardiomyopathy.
Section Outline:
CONTRAINDICATIONSA history of allergy to NAC is a relative contraindication.
ADVERSE EFFECTS
- Oral administration. Nausea and vomiting are common; diarrhea and rash occur rarely.
- Intravenous administration. Allergic reactions ranging from rash to anaphylaxis occur rarely.
[
Show Section Outline]
ORAL DOSAGE
- The loading dose for adult and pediatric patients is 140 mg/kg orally.
- The maintenance dose for adult and pediatric patients is 70 mg/kg orally every 4 hours for 17 additional doses (72 hour protocol).
- Health-care provider should be aware that some poison centers recommend a truncated treatment course under special conditions.
- To administer NAC, 10% or 20% NAC solution is diluted in water, juice, or soda to a 5% solution.
- Use of ice and a straw may diminish the unpleasant taste and odor, making NAC more palatable to drink.
- If the patient is unable to drink, NAC may be administered via small-bore nasogastric tube.
- If the patient vomits within 1 hour of any dose, the full dose should be readministered.
- Aggressive anti-emetic therapy may be required for repetitive vomiting.
- Adult regimen is metoclopramide 1.0 to 2.0 mg/kg intravenously, plus prochlorperazine 10 mg or droperidol 2.5 to 5.0 mg, administered intravenously.
- Diphenhydramine 25 to 50 mg intravenously may be added to this regimen.
- Ondansetron 8 mg intravenously may be used if the above medications fail.
- If liver or renal injury is not improving, NAC should be continued beyond the standard 72 hours (oral) or 48 hours (intravenous) protocol, until improvement of liver and renal function occurs.
ADMINISTRATION OF ORAL FORMULATION BY THE INTRAVENOUS ROUTE
- Occasionally, oral administration cannot be used because of intractable vomiting despite antiemetic therapy or medical conditions that contraindicate oral intake (gastrointestinal hemorrhage, acute abdomen, intestinal obstruction, or severe oral or esophagogastric burns).
- An intravenous NAC preparation is not available in the United States.
- Because the commercially available NAC solution is sterile (intended for nebulized or oral treatment), however, patients may be safely treated with the oral NAC formulation administered via the intravenous route.
- The Rocky Mountain Poison and Drug Center can provide medical consultation to health-care providers on the method of administration (800-525-6115).
- The United States intravenous protocol is as follows.
- If possible, informed consent should be obtained.
- Intravenous NAC should be administered in a monitored setting such as an emergency department or ICU.
- The loading dose is 140 mg/kg, followed by maintenance doses of 70 mg/kg intravenously every 4 hours for 12 additional doses (48-hour protocol).
- Some poison centers recommend truncated treatment course under special conditions.
- The 10% or 20% NAC solution should be diluted to 5% concentration with D5W.
- Each dose is infused over 30 to 60 minutes through a 0.2-µm in-line filter. Faster rates of infusion are associated with more frequent adverse effects.
- A 20-hour intravenous NAC protocol is approved for use in the United Kingdom, but it is not commonly used in the United States. The NAC dosage for the 20-hour protocol is 150 mg/kg in 200 ml D5W infused over 15 minutes, followed by 50 mg/kg in 500 ml D5W infused over 4 hours, followed by 100 mg/kg in 1 L D5W over 16 hours.
TREATMENT OF REACTIONS DURING NAC INFUSION
Dermatologic Reactions
- The infusion should be temporarily discontinued.
- Development of rash does not contraindicate further use of NAC.
- Diphenhydramine should be administered intravenously: adult, 25 to 50 mg; pediatric, 1 to 2 mg/kg up to adult dose.
- The rash typically resolves within 1 hour.
- The intravenous NAC may be restarted.
- Subsequent doses may require pretreatment with diphenhydramine.
Anaphylactoid Reaction (Hypotension, Angioedema, Bronchospasm)
- NAC should be discontinued and the anaphylactoid reaction treated in the standard manner.
- Further use of NAC intravenously is relatively contraindicated. Consultation with a poison center is recommended.
Section Outline:
ICD-9-CM 965Poisoning by analgesics, antipyretics, and antirheumatics.
See Also: SECTION IV, Acetaminophen—Acute Single Ingestion and Acetaminophen—Repeated Ingestion chapters.
RECOMMENDED READING
Dawson AH, Henry DA, Macewen J. Adverse reactions to N-acetylcysteine during treatment for paracetamol poisoning. Med J Aust 1989;150:329-331.
Flanagan RJ, Meredith TJ. Use of N-acetylcysteine in clinical toxicity. Am J Med 1991;91:131S-139S.
Smilkstein MJ, Bronstein AC, Linden C, et al. Acetaminophen overdose: a 48 hour intravenous N-acetylcysteine protocol. Ann Emerg Med 1991;20:1058-1063.
Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose: analysis of the national multicenter study (1976 to 1985). N Engl J Med 1988;319:1557-1562.
Yip L, Dart RC, Hurlbut KM. Intravenous administration of oral N-acetylcysteine. Crit Care Med 1998;26:40-43.
Author: Rivka S. Horowitz
Reviewer: Richard C. Dart

