[
Show Section Outline]
DESCRIPTION
Acetaminophen is an oral analgesic available in many over-the-counter and prescription medications. It is also known as paracetamol in many countries.
FORMS AND USES
- Numerous brands contain acetaminophen alone, whereas others are combination products.
- Acetaminophen in all formulations is used for temporary relief of mild to moderate pain and for temperature reduction in febrile patients.
- Regular formulation acetaminophen. Dosage for adults and children 12 years and older is as follows:
- Oral: 325 to 650 mg every 4 to 6 hours, not to exceed 4 g in 24 hours; single doses of 1 g may be used in adults.
- Rectal: 325 to 650 mg every 4 to 6 hours as needed, not to exceed 4 g in 24 hours.
- Tylenol Arthritis Extended Relief Caplet is a bilayer of 325 mg immediate-release acetaminophen and 325 mg slow-release acetaminophen.
- Adults and children 12 years and older: 1.3 g every 8 hours as needed, not to exceed 3.9 g in 24 hours.
- Pediatric suspension, elixir, or drops (oral or rectal): 10 to 15 mg/kg every 4 to 6 hours, not to exceed 90 mg/kg in 24 hours.
- If the product packaging is available, the health-care professional should check the product label to determine whether the main ingredient is acetaminophen or acetylsalicylic acid (aspirin) and to identify other active constituents such as decongestants, stimulants, or opioids.
TOXIC DOSE
An acute ingestion of greater than 15 g in an adult often produces liver injury. The pediatric toxic dose of a single ingestion is unknown.
PATHOPHYSIOLOGY
- Acetaminophen is primarily metabolized to nontoxic products in the liver.
- An additional metabolic pathway, involving cytochrome P450, produces a toxic metabolite, N-acetyl-p-benzoquinoneimine (NAPQI), which causes liver injury if not detoxified.
- With therapeutic dosing, NAPQI is detoxified by liver glutathione and no liver injury occurs.
- However, high blood levels of acetaminophen deplete glutathione stores and allow NAPQI to bind to liver cells, producing cell injury and death.
- Acetaminophen toxicity also stimulates the release of inflammatory mediators such as cytokines, which further contribute to liver injury.
EPIDEMIOLOGY
- Intentional overdose is common.
- Toxicity is typically mild if the patient is treated early.
- Death is unusual, developing in patients who present after 24 hours or in whom treatment is mistakenly withheld.
- "Chronic" acetaminophen toxicity may result from repetitive, supratherapeutic dosing; such poisonings are usually unintentional and occur in adults with acute, persistent pain syndromes or in persistently febrile infants. Adults who ingest greater than 4 g/day for more than 1 day, and infants less than 2 years of age who are given more than 150 mg/kg/day for more than 1 day, may be at increased risk.
CAUSES
- Ingestion is usually with suicidal intent.
- Child neglect or abuse should be considered if the patient is less than 1 year of age, suicide attempt if the patient is older than 6 years of age.
DRUG AND DISEASE INTERACTIONS
- Adults with malnutrition or underlying liver disease (e.g., alcoholics) who ingest an overdose amount may be more susceptible to liver toxicity.
- Chronic ingestion of medications which induce cytochrome P450 enzymes, such as isoniazid and carbamazepine, may potentiate acetaminophen toxicity.
PREGNANCY AND LACTATION
- US FDA Pregnancy Category B. Animal studies indicate no fetal risk and there are no controlled human studies, or animal studies show an adverse fetal effect but well-controlled studies in women do not.
- Acetaminophen crosses the placenta, but documented fetal death or hepatotoxicity from maternal overdose is rare.
- In the absence of fetal distress, induction of labor or termination of pregnancy based on maternal acetaminophen toxicity is not indicated.
- Preliminary evidence indicates that the antidote, N-acetylcysteine (NAC), crosses the placenta and should be administered to a pregnant woman with the same indications as patients who are not pregnant.
- An infant born to a mother with acetaminophen toxicity should receive a 48-hour course of intravenous NAC.
Section Outline:
[
Show Section Outline]
DIFFERENTIAL DIAGNOSIS
- Toxicologic agents that cause nausea and vomiting for several hours followed by right upper quadrant pain and elevated liver function tests include amanita mushroom, carbamazepine, valproic acid, phenytoin, methyldopa, isoniazid, carbon tetrachloride, halothane, disulfiram, pennyroyal, procainamide, pyrrolizidine alkaloids, and methotrexate, among others.
- Nontoxicologic causes include biliary tract disease and viral or alcoholic hepatitis.
SIGNS AND SYMPTOMS
- Acute overdose is usually characterized by nausea, vomiting, and diffuse abdominal pain, beginning within hours after overdose and resolving within 24 hours.
- Right upper quadrant pain and laboratory evidence of liver injury usually develops between 24 to 48 hours.
- Severe toxicity may produce fulminant hepatic failure 3 to 5 days postingestion.
- Patients with "chronic" overdose resulting from repetitive, supratherapeutic dosing usually present with liver injury already established [elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), prothrombin time, and bilirubin levels].
Vital Signs
Vital signs are usually normal, although patients with dehydration from vomiting or anorexia may have tachycardia.
Dermatologic
Jaundice may occur if hepatic injury develops.
Gastrointestinal
- Nausea and vomiting are common over the first 24 hours after ingestion.
- Pancreatitis occurs rarely, 3 to 5 days after ingestion.
Hepatic
- Liver injury ranges from mild, asymptomatic disease to fulminant hepatic failure.
- Right upper quadrant pain and abnormal liver function tests typically develop between 24 and 48 hours postingestion, although liver injury may occasionally develop earlier.
Renal
Acute renal failure may develop 3 to 5 days postingestion and may occur independent of hepatic injury.
Hematologic
Thrombocytopenia has been reported.
Fluids and Electrolytes
Dehydration may occur from intractable vomiting and anorexia.
Neurologic
Hepatic encephalopathy and coma may complicate hepatic failure.
Acid-Base
Increased anion gap metabolic acidosis may occur after massive acute overdose, but more common causes of metabolic acidosis must be sought.
PROCEDURES AND LABORATORY TESTS
Essential Tests
Serum acetaminophen level drawn between 4 and 24 hours after ingestion of acute overdose. The optimal level is drawn at 4 hours after ingestion or as soon after 4 hours as possible.
- The serum level should be plotted on the Rumack-Matthew nomogram (see figure).
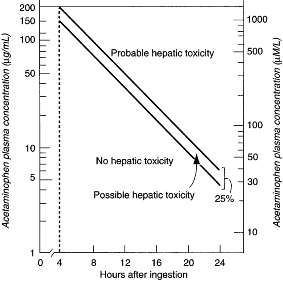
Semilogarithmic plot of plasma acetaminophen levels versus time. (Modified and reproduced, with permission, from Rumack BH, Matthew H. Acetaminophen poisoning and toxicity. Pediatrics 1975;55:871.)
- If the level is above the "possible toxicity" level (the lower of the two lines), NAC is administered.
- The Rumack-Matthew nomogram cannot be used when the time of ingestion is uncertain, if the ingestion occurred more than 24 hours before presentation, or if repeated ingestion has occurred.
- Tylenol Arthritis Extended Relief does not fulfill the assumptions of the Rumack-Matthew nomogram.
- The nomogram assumes that acetaminophen is completely absorbed within 4 hours of ingestion, but Tylenol Arthritis Extended Relief may be absorbed beyond this time.
- Thus, if the initial serum acetaminophen level falls into the nontoxic range, a second level should be obtained 4 to 6 hours later.
- If either level is on or above the nomogram's possible toxicity line, a full course of NAC should be administered.
- NAC therapy should be initiated empirically if the serum acetaminophen level is delayed or the patient presents more than 8 hours after ingestion. In these situations, the delayed administration of NAC may allow liver injury to occur, and NAC should be administered until it is found that the serum acetaminophen level is nontoxic.
- NAC can be discontinued if the acetaminophen level is later found to be nontoxic on the Rumack-Matthew nomogram.
- Serum electrolytes, BUN, creatinine, glucose
- Massive acetaminophen ingestion may produce metabolic acidosis 8 to 12 hours after ingestion.
- Hepatic failure may produce acidosis, electrolyte abnormalities, and hypoglycemia.
- Prothrombin time or international normalized ratio (PT/INR), AST, ALT, and bilirubin
- Elevated PT is the earliest sign of acetaminophen toxicity.
- Liver enzymes begin to increase at about 24 hours and peak at 72 hours.
Recommended Tests
- Serum ammonia is elevated in hepatic encephalopathy.
- Arterial blood gases are used to assess the severity of acidemia.
- ECG and aspirin level in overdose setting are used to detect occult overdose.
Not Recommended Tests
- Once NAC therapy has been initiated, repeated serum acetaminophen levels are not useful.
- The only exception is for Tylenol Arthritis Extended Relief, as described above.
Section Outline:
[
Show Section Outline]
- Treatment focuses on prevention of absorption, control of emesis, and administration of NAC within 8 hours of ingestion (as indicated by the Rumack-Matthew nomogram) if potentially toxic ingestion has occurred.
- Aggressive antiemetic therapy is important for patients with vomiting who require NAC therapy.
- The dose and time of exposure should be determined for all substances involved.
DIRECTING PATIENT COURSE
The health-care professional should call the poison control center when:
- Metabolic acidosis, renal insufficiency, encephalopathy, or hepatic failure develop.
- Ingestion involves Tylenol Arthritis Extended Relief Caplets, or patients presenting more than 24 hours postingestion.
- Oral NAC therapy cannot be successfully administered.
- Repeated (chronic) acetaminophen ingestion is suspected.
- Toxic effects are not consistent with acetaminophen poisoning.
- Coingestant, drug interaction, or underlying disease presents an unusual problem.
Patients should be referred to a health-care facility when:
- Attempted suicide or homicide is possible.
- Patient or caregiver seems unreliable.
- Toxic effects are apparent.
- Children with estimated ingestion greater than 150 mg/kg should be referred to an emergency department for charcoal and a 4-hour serum acetaminophen level.
- Coingestant, drug interaction, or underlying disease presents an unusual problem.
Admission Considerations
Inpatient management is warranted for:
- Patients who require treatment with NAC, present 24 to 36 hours after ingestion, or have an uncertain time of ingestion but have a measurable acetaminophen level or a history of acetaminophen overdose given by others.
- Patients with chronic toxicity.
Consultation with a liver transplant center is recommended when the following signs of poor prognosis are present:
- Arterial pH is less than 7.3.
- Encephalopathy is rated at grade III or IV.
- Creatinine level is greater than 3.4 mg/dl.
- PT is greater than 35 to 40 seconds, or PT is increasing rapidly.
DECONTAMINATION
Out of Hospital
Ipecac should be administered to induce emesis within 1 hour of ingestion for alert pediatric or adult patient, if health-care evaluation will be delayed.
In Hospital
- Ipecac should be administered to induce emesis within 1 hour of ingestion for the alert patient who is too small to have effective gastric lavage.
- Gastric lavage should be performed in pediatric (tube size 24-32 French) or adult (tube size 36-42 French) patients for large ingestion presenting within 1 hour of ingestion or if serious effects are present.
- One dose of activated charcoal (1-2 g/kg) should be administered without a cathartic if a substantial ingestion has occurred within the previous few hours.
- Simultaneous administration of activated charcoal and NAC will not affect outcome.
ANTIDOTES
NAC is a very effective antidote for acute acetaminophen ingestion.
Indications
- An acute overdose has occurred, and the serum acetaminophen level, drawn between 4 and 24 hours after ingestion, is plotted on or above the possible toxicity line (lower of the two lines) on the Rumack-Matthew nomogram.
- An acute ingestion has occurred, but the time of ingestion is uncertain or unknown.
- An acute overdose is possible and elevated liver function tests are already present. An increase in AST or ALT is considered to be due to an acetaminophen injury until proven otherwise.
- A patient presents with an acute overdose more than 48 hours after ingestion; the patient should receive NAC if liver enzymes are abnormal, but NAC is not needed if liver enzymes are normal.
- Because liver toxicity may not become apparent until 24 to 48 hours postingestion, patients with a history of a single acute ingestion 24 to 36 hours prior to presentation should receive NAC therapy regardless of whether measurable acetaminophen is present or whether liver function tests are normal.
- Chronic toxicity also indicates use (see SECTION IV, Acetaminophen—Repeated Ingestion chapter).
Contraindications
History of anaphylactic reaction to NAC precludes use.
Method of Administration
- Water, juice, or soda is used to dilute a 10% or 20% NAC solution to a 5% solution.
- A loading dose of 140 mg/kg is administered orally, followed by 17 maintenance doses of 70 mg/kg every 4 hours.
- If the patient vomits NAC within 1 hour of any dose, the dose is repeated.
- Administering NAC oral formulation via an intravenous route. On rare occasions, patients with acetaminophen toxicity cannot tolerate oral NAC; such patients may be treated with the oral formulation administered via the intravenous route.
- NAC is continued beyond 72 hours (oral) or 48 hours (intravenous protocol) if the liver or renal injury is not improving.
- Treatment of alcoholic patients is the same as for nonalcoholic patients.
Adverse Effects
- NAC has an unpleasant smell and taste.
- Urticaria and papular rashes occur rarely.
- Rarely, intravenous administration of NAC overdose has caused life-threatening allergic reactions.
ADJUNCTIVE TREATMENT
- Antiemetic therapy. Patients may require aggressive antiemetic therapy to ensure that NAC is retained.
- Therapies not recommended
- Because PT is an important prognostic indicator, vitamin K1 or fresh frozen plasma administration is not recommended to correct PT of less than 40 seconds unless frank bleeding develops.
- Cimetidine is an unproven therapy and is not recommended.
Section Outline:
[
Show Section Outline]
PATIENT MONITORING
If the patient requires NAC treatment, PT or INR, AST, ALT, bilirubin, electrolytes, BUN, creatinine, and glucose should be repeated daily.
EXPECTED COURSE AND PROGNOSIS
Most patients who are treated with NAC recover without sequelae even if liver injury complicates the course. Prognosis is related to the time to treatment with NAC. Mild hepatotoxicity, or none, is expected if treated within 8 hours of ingestion, but the likelihood of liver injury increases if NAC is administered more than 8 hours after overdose. Fulminant hepatic failure may develop if NAC is started more than 24 hours after ingestion.
POSSIBLE COMPLICATIONS
- Fulminant hepatic failure is the main cause of death from acetaminophen toxicity; a liver transplant may be life saving.
- Renal injury occurs rarely, developing 3 to 5 days postingestion and after the onset of hepatotoxicity.
- Renal failure is usually reversible but may last for several weeks and require hemodialysis.
DISCHARGE CRITERIA/INSTRUCTIONS
- From the emergency department. Patients may be discharged if the time of ingestion is certain, the serum acetaminophen level is below the possible toxicity level, and a psychiatric evaluation has been performed.
- From the hospital. Patients may be discharged after a full course of NAC, if liver and renal function tests are normal or improving, and after a psychiatric evaluation, if needed.
PATIENT EDUCATION
- The health-care professional should educate patients on the potential dangers of over-the-counter medications.
- Patients should be cautioned that simultaneous use of more than one acetaminophen product may lead to inadvertent overdose.
- Substitution of inappropriate formulation (e.g., use of an adult acetaminophen suppository in a child instead of a pediatric one) may result in toxicity.
Section Outline:
DIAGNOSISSigns of poisoning may be minimal and overlooked during the first 24 hours.
TREATMENT
Failure to treat vomiting aggressively may result in substantial delays in NAC treatment and less favorable patient outcome.
ICD-9-CM 965.4Poisoning by aromatic analgesics (acetaminophen).
See Also: SECTION III, N-acetylcysteine chapter; and SECTION IV, Acetaminophen—Repeated (Chronic) Ingestion chapter.
RECOMMENDED READING
Keays R, Harrison P, Wendon, J, et al. Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: a prospective controlled trial. Br Med J 1991;303:1026-1029.
O'Grady JG, Alexander G, Hayllar KM, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology 1989;97:439-445.
Rumack BH, Matthew H. Acetaminophen poisoning and toxicity. Pediatrics 1975;55:871-876.
Smilkstein MJ, Knapp GL, Kulig KW, et al. Efficacy of oral N-acetylcysteine in the treatment of APAP overdose: analysis of the national multicenter study (1976 to 1985). N Engl J Med 1988;319:1557-1562.
Yip L, Dart RC, Hurlbut KM. Intravenous administration of oral N-acetylcysteine. Crit Care Med 1998;26:40-43.
Author: Rivka S. Horowitz
Reviewer: Richard C. Dart

