Clinical classification or pretreatment clinical classification, designated TNM or cTNM, is essential for selecting and evaluating therapy. It is based on the evidence found before treatment, including the results of history and physical examination, imaging studies (e.g., computed tomography [CT] and positron emission tomography [PET]), laboratory tests, and staging procedures such as bronchoscopy or esophagoscopy with or without ultrasound-guided biopsy (e.g., using endobronchial ultrasound [EBUS] or endoscopic ultrasound [EUS]), mediastinoscopy, mediastinotomy, extended cervical mediastinoscopy, thoracentesis, pleural biopsy, pericardioscopy, thoracoscopy, and video-assisted thoracoscopic surgery, as well as exploratory thoracotomy.
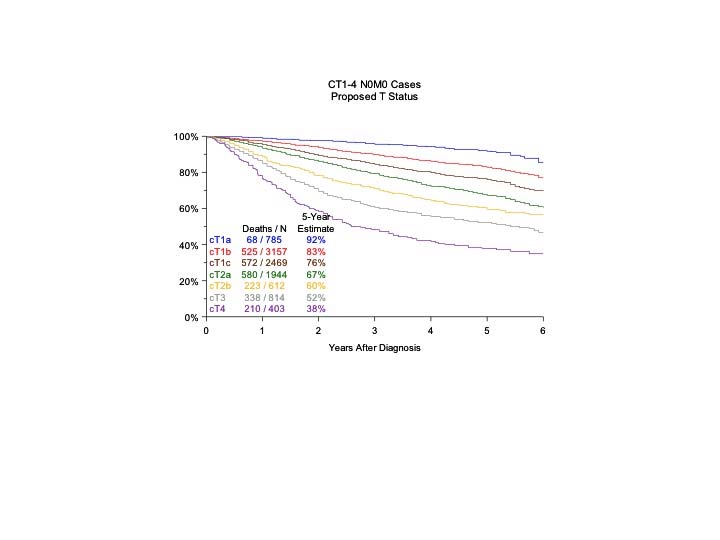
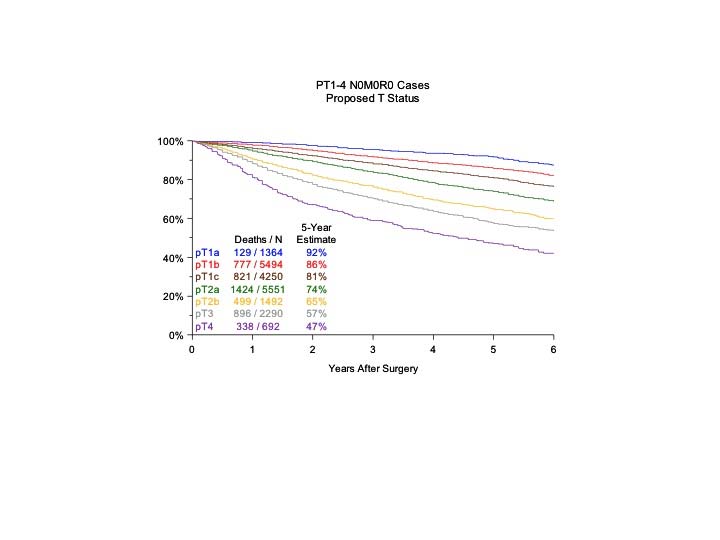
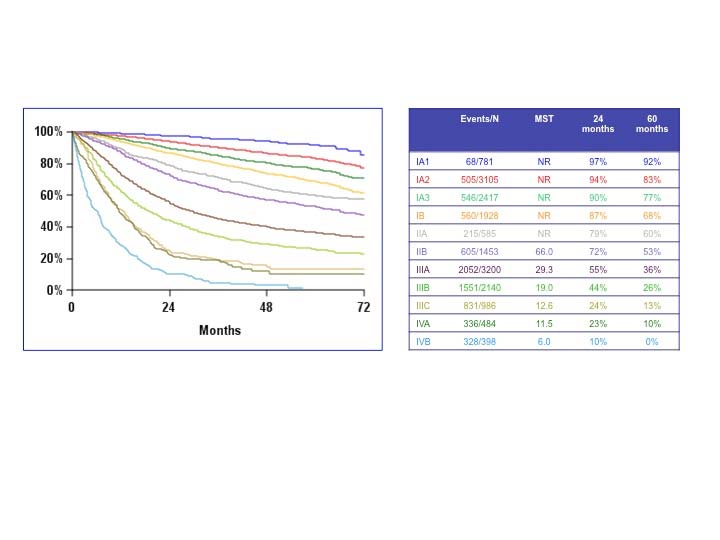
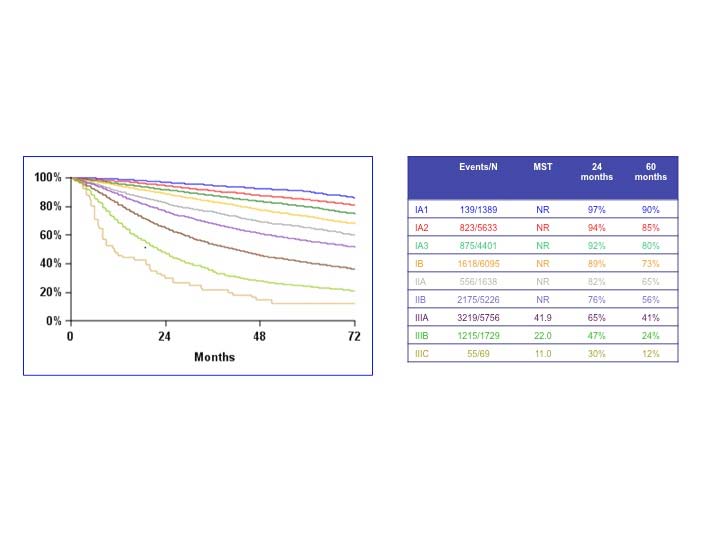
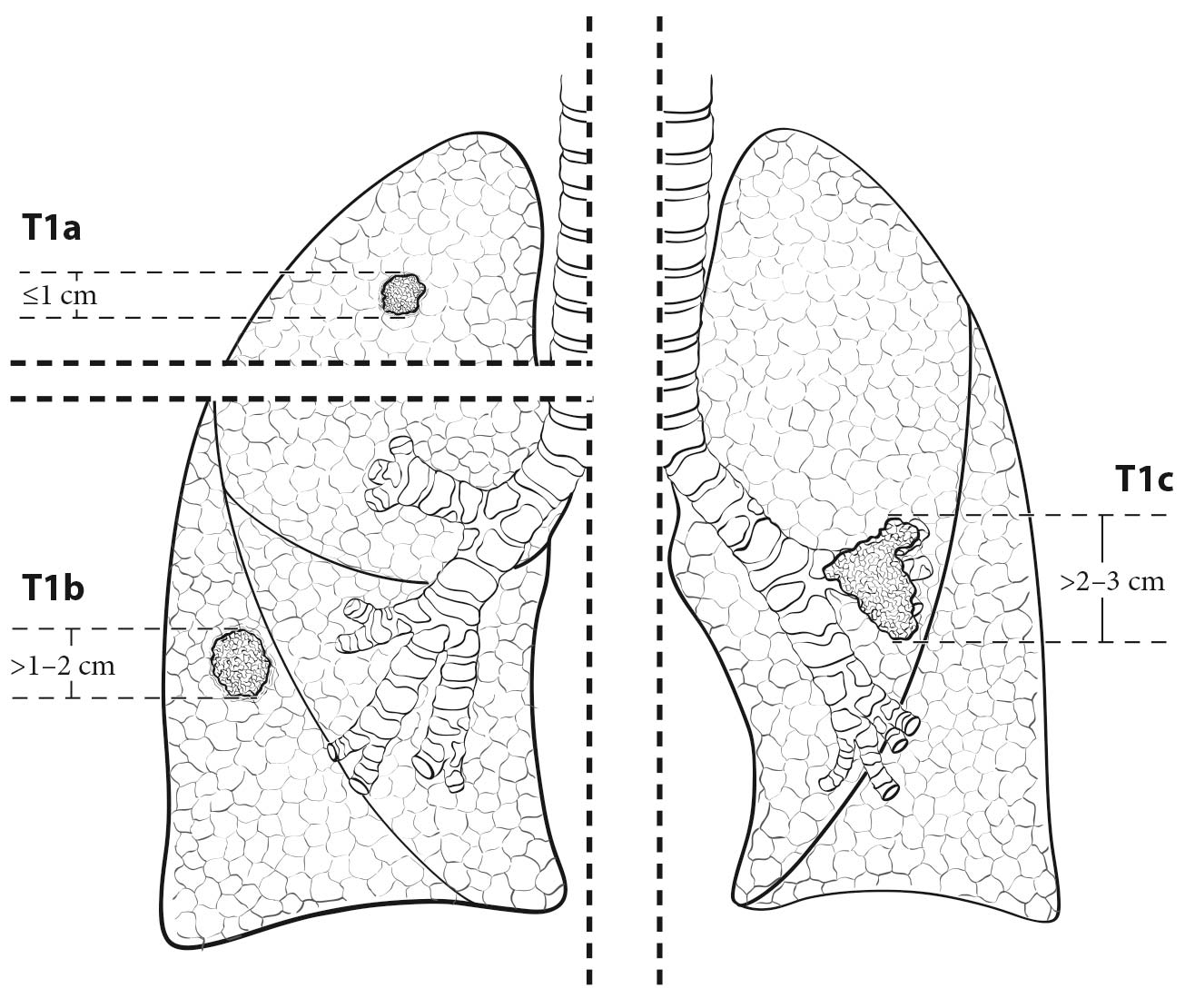
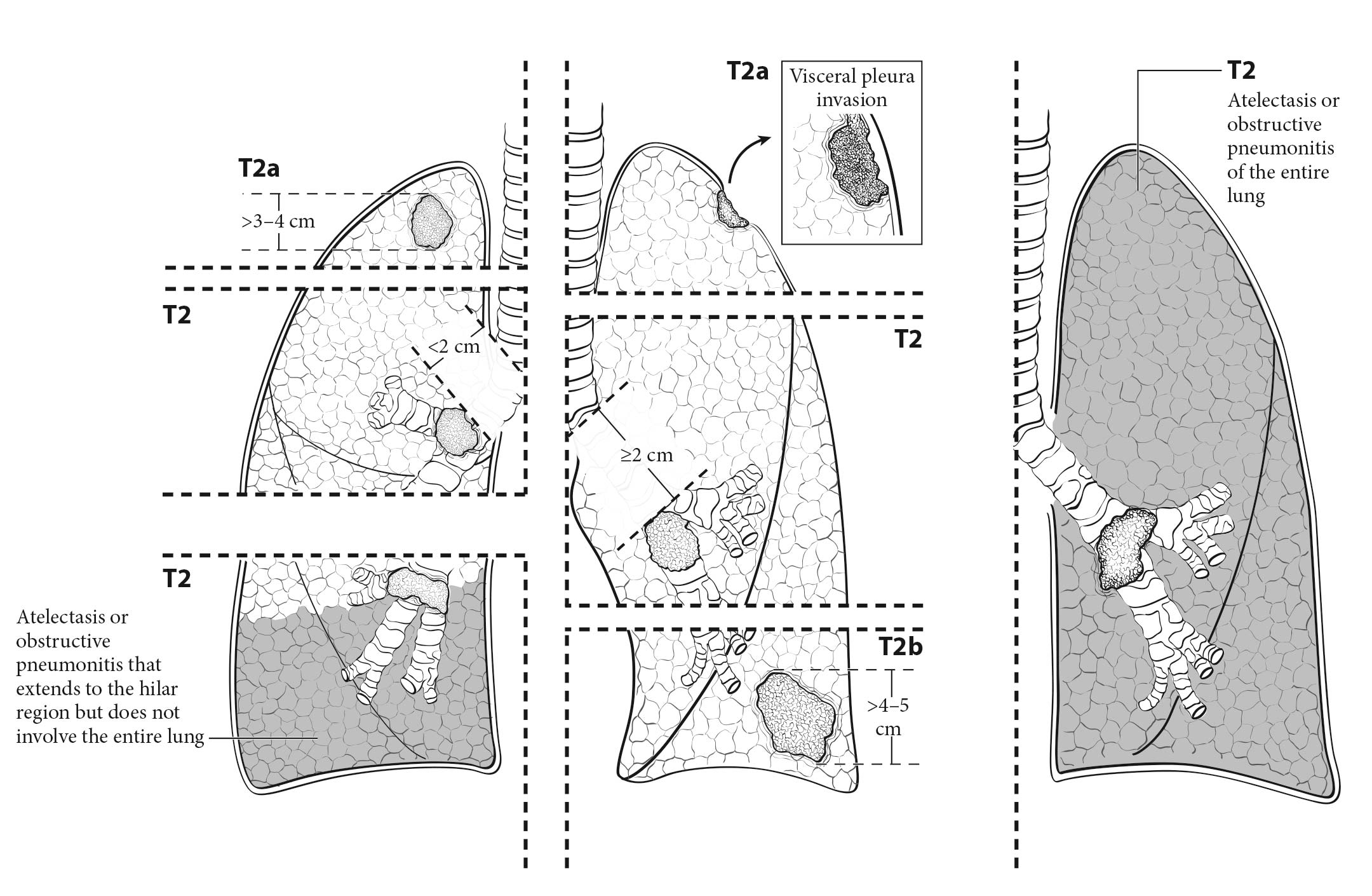
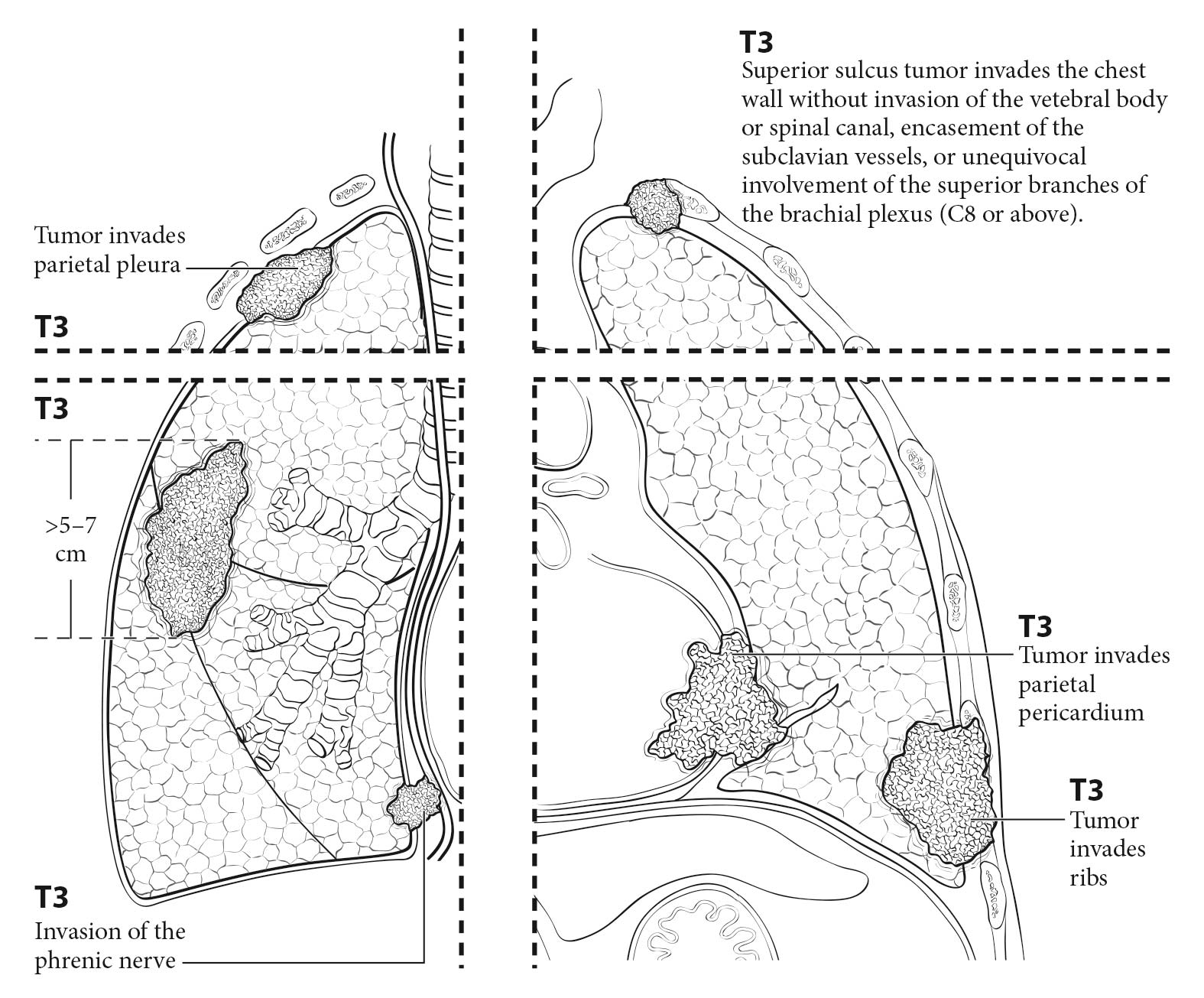
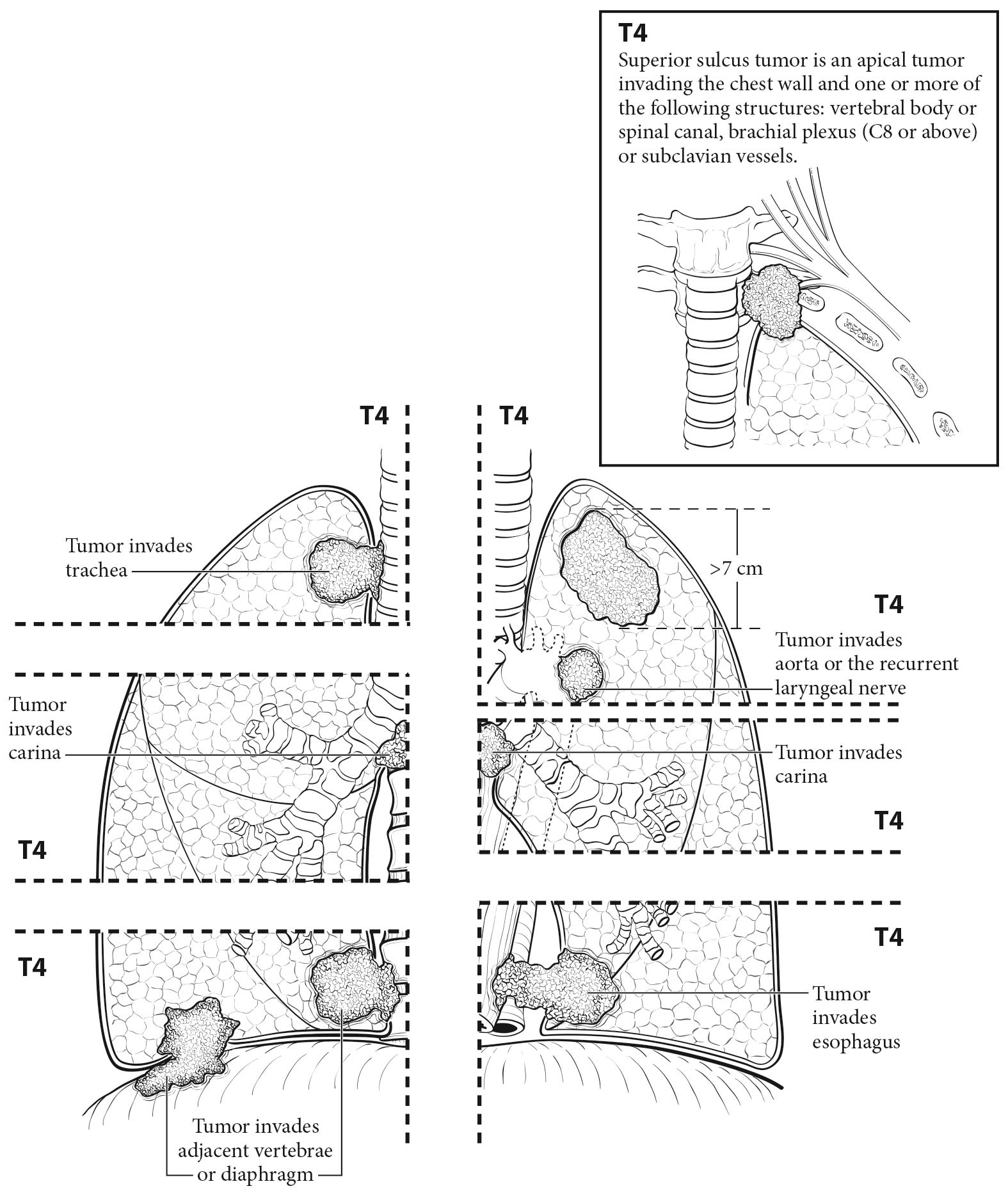
The analyses of the new IASLC database concerning the primary tumor (T) component of the TNM classification revealed that tumor size has more prognostic relevance than was shown in previous editions. Each centimeter increase in size, from less than 1 cm to up to 5 cm, separates tumors of significantly different prognosis, and larger tumors have a worse prognosis than their assigned T categories in the 7th Edition. Regarding the descriptors of tumor invasion, the prognosis worsens if tumor invasion is more central. On the other hand , endobronchial tumors less than or equal to 2 cm from the carina, but not involving the carina, and those causing complete atelectasis or pneumonitis do not have a worse prognosis than those more than 2 cm from the carina or those causing partial atelectasis and pneumonitis. Figure 36.2 shows the survival curves of the new T categories for clinically staged tumors with no nodal involvement and no metastases, and Figure 36.3 shows the survival curves for completely resected tumors with no nodal involvement and no metastases. In these graphs, the survival curves separate completely, with no overlapping or crossing; and survival differences between T3 and T4 tumors with no nodal involvement or metastasis are statistically significant, which was not the case in previous editions of the TNM classification.3
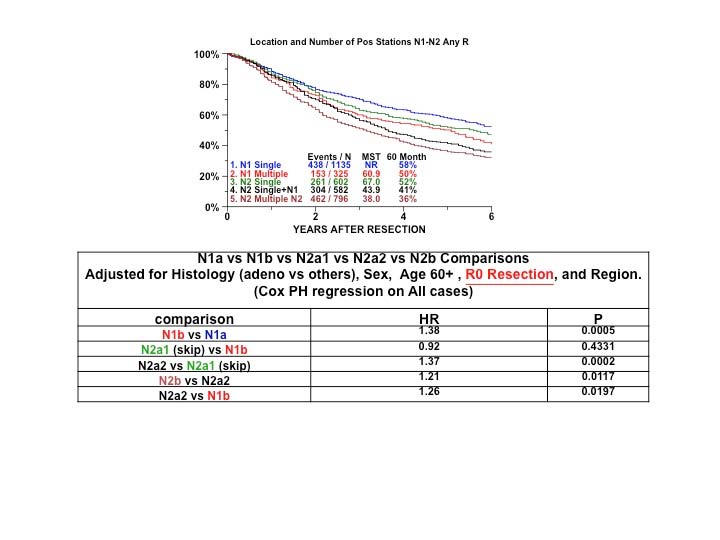
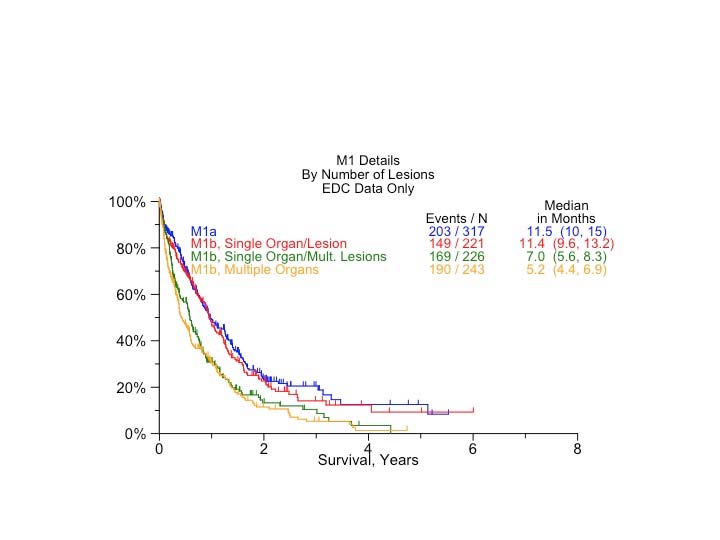
Classification of lung cancers with multiple lesions poses some problems, because the rules are sometimes ambiguous and their application may be interpreted differently in each situation. Therefore, a special subcommittee of the IASLC Staging and Prognostic Factors Committee studied the problem and made some recommendations regarding the uniform use of the classification rules depending on the pattern of disease. The subcommittee established four disease patterns: second primary tumors, lung cancers with separate tumor nodules of the same histopathological type, multiple tumors with predominant ground-glass features on CT and a lepidic pattern on pathological examination, and , finally, diffuse pneumonic-type lung cancer.16 Three in-depth articles expand the rationale for applying the classification rules to each disease pattern.17-19
The aforementioned recommendations for classifying the different patterns of disease are the result of a multidisciplinary and international consensus as well as a thorough literature review and statistical analysis of data from the IASLC database regarding separate tumor nodules. These suggestions are meant to minimize ambiguity and to serve as a guide in classifying these tumors uniformly.
Imaging
Medical history (e.g., family history of lung cancer or of any cancer, smoking history, exposure to asbestos or radon, history of passive smoking, presence of respiratory symptoms or chest pain) and physical examination findings (e.g., peripheral adenopathy, abnormal breath sounds, superior vena cava syndrome, hemoptysis, hepatomegaly) will prompt the request for a series of explorations to confirm or rule out lung cancer. There are many imaging techniques and invasive procedures that may be used to diagnose and stage lung cancer. Whenever possible, they should be performed sequentially and with an increasing degree of invasiveness.
The IASLC recommends a three-step protocol to rationalize the use of staging procedures. Step I includes medical history and physical examination, as well as plain radiographs of the chest and blood tests (hemoglobin, leukocytes, platelets, alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, calcium, and albumin). Step II includes more complex investigations, such as contrast-enhanced CT scan of the chest and upper abdomen, bone scan, PET scan, brain CT, and bronchoscopy. Step III includes the more invasive procedures, including surgical exploration of the mediastinum (mediastinoscopy, extended cervical mediastinoscopy, mediastinotomy, video-assisted mediastinal lymphadenectomy, transcervical extended mediastinal lymphadenectomy), the pleural space (thoracentesis, percutaneous needle biopsy, thoracoscopy, video-assisted thoracoscopic surgery), or the pericardium (pericardiocentesis, pericardioscopy).20
Posteroanterior and lateral chest radiography usually is the first imaging technique requested for a patient with suspected lung cancer. The minimal information to be extracted from chest X-rays is:
- Tumor size (to assign a T category based on size)
- Lobar and segmental location of the tumor
- Presence of atelectasis and its extent (lobar or complete; cT2)
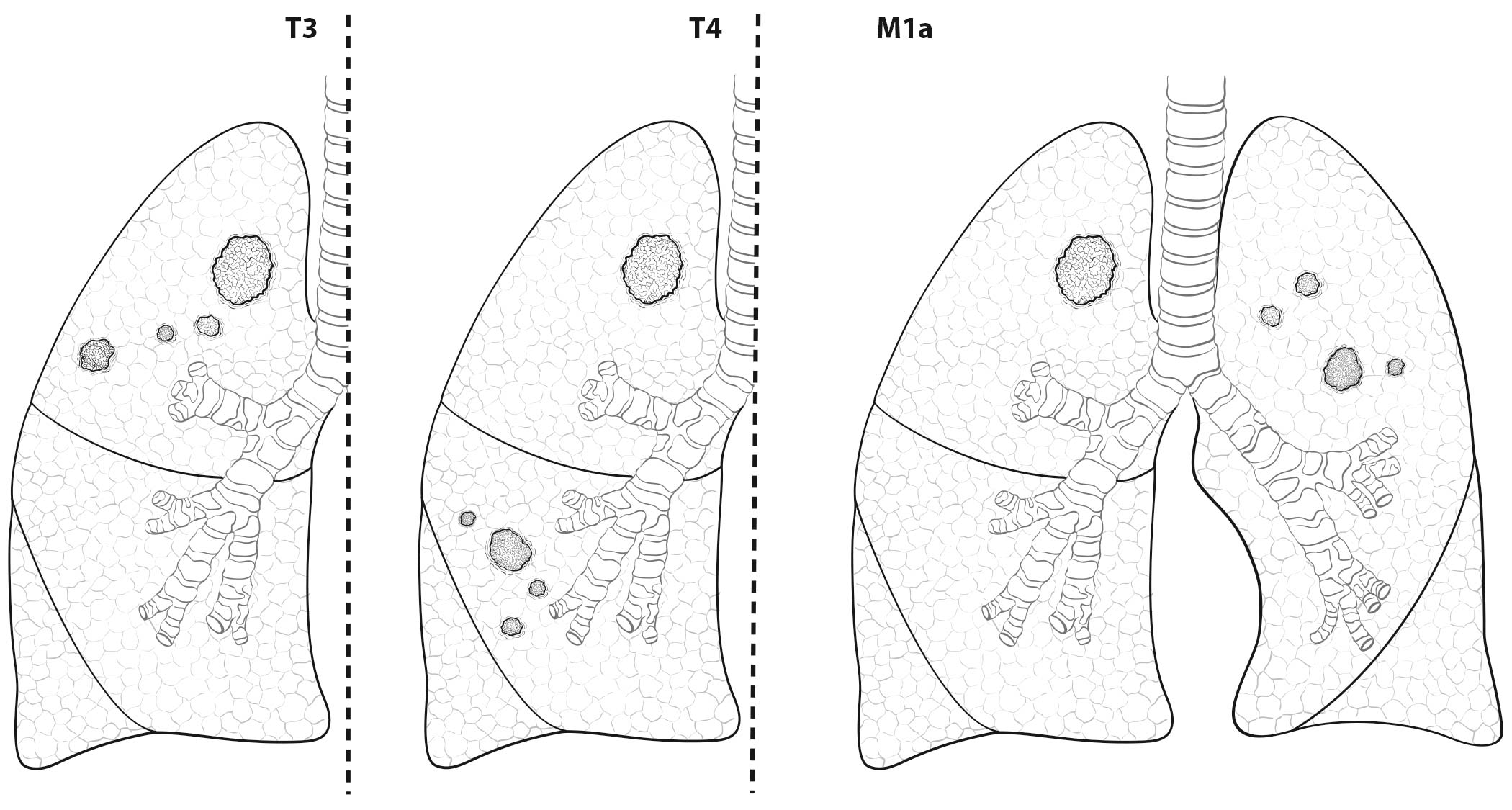
- Presence of separate tumor nodules (cT3, cT4, or cM1a)
- Evidence of lymphangitic carcinomatosis (cLy0: no radiologic evidence of lymphangitic carcinomatosis; cLy1: radiologic evidence of lymphangitic carcinomatosis confined to the area of the primary tumor; cLy2: lymphangitic carcinomatosis at a distance from the primary tumor but confined to the same lobe; cLy3: presence of lymphangitis in other ipsilateral lobes; cLy4: lymphangitis affecting the contralateral lung)
- Relation of the primary tumor to the chest wall (contact or bone destruction [cT3]) or the mediastinum (elevated diaphragm may indicate invasion of the phrenic nerve [cT3])
- Nodal spread: enlarged hili and abnormal mediastinum may indicate cN1, cN2, or cN3 disease
- Intrathoracic spread: the presence of pleural or pericardial effusions (cM1a)
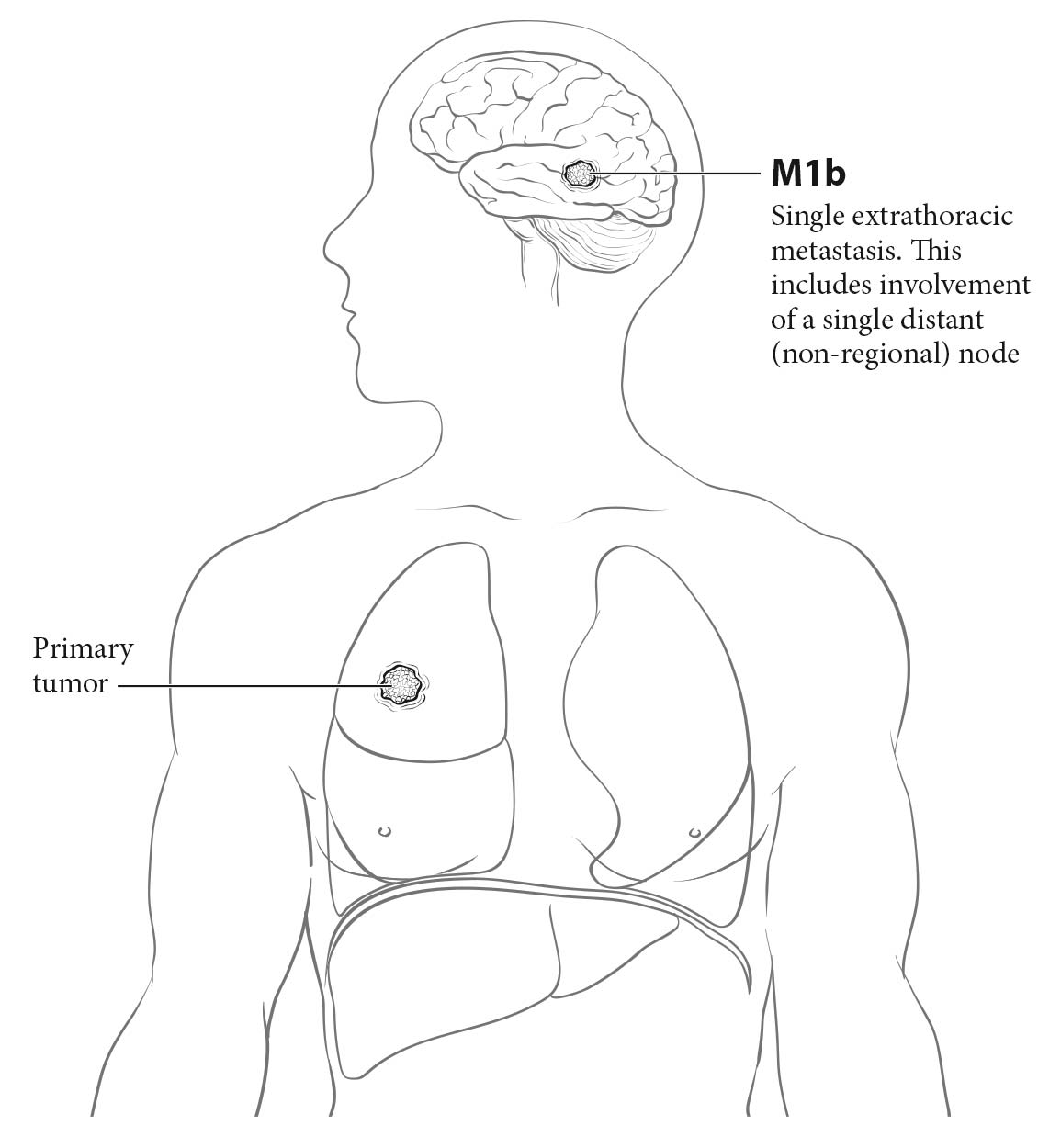
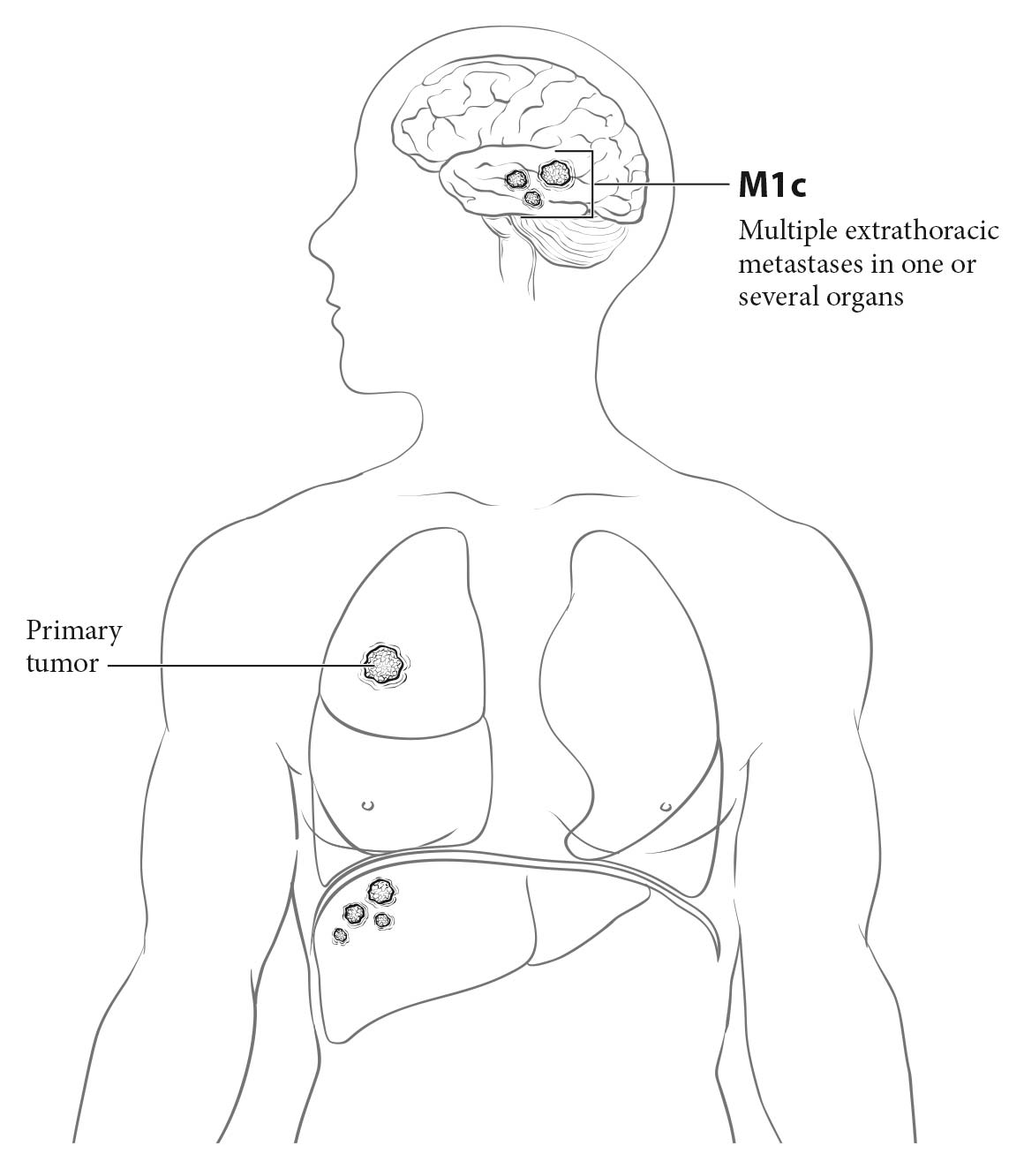
- Extrathoracic spread: the integrity or involvement of the bones visible on chest X-rays—the ribs, the sternum, both scapulae, the vertebral column, the shoulder joint, and most of the length of both humeri; masses in the soft tissues of the chest wall (cM1b or cM1c)
Contrast CT of the chest and upper abdomen to include the liver and both adrenal gland s is recommended for patients who are cand idates for radical treatment, whether it be surgery, primarily or after induction, or definitive chemoradiation.21 CT should confirm and refine the information obtained from the chest X-rays:
- Tumor size in its greatest dimension, which may be assessed by axial, coronal, or sagittal measurement; therefore, if technically possible, all the different projections should be studied to determine tumor size. Lung CT window display settings should be used when assessing images for tumor size.
- Lobar and segmental location
- Presence of atelectasis (partial or total; cT2) and endobronchial lesions
- Presence of separate solid tumor nodules (cT3, cT4, or cM1a) and presence of part-solid lesions
- Nodal spread: hilar enlargement suggests cN1 disease if it is ipsilateral to the primary tumor or cN3 disease if it is contralateral. Nodes should be measured in their short axis; those larger than 1 cm in the short axis are considered abnormal and suggest metastatic involvement. Enlarged mediastinal lymph nodes may indicate cN2 disease if they are ipsilateral or subcarinal or cN3 disease if they are contralateral or supraclavicular. The number of nodal stations involved should be determined; bulky disease should be identified if present. Mediastinal CT window display settings should be used when assessing images for mediastinal structures, pleural or pericardial effusions, etc.
- Evidence of lymphangitic carcinomatosis (cLy0, cLy1, cLy2, cLy3, and cLy4 as defined earlier)
- Intrathoracic spread: pleural and pericardial effusion or nodules (cM1a)
- Extrathoracic spread: bone lesions, soft tissue masses, adrenal masses, and liver nodules may indicate cM1b disease if single or cM1c disease if multiple
CT is important for assessing the size and location of any enlarged mediastinal lymph nodes present, because in the absence of metastasis, they are the strongest indicators of prognosis. In a review of 7,368 patients with a median prevalence of mediastinal nodal disease of 30%, staging values of chest CT were as follows: sensitivity, 0.55; specificity, 0.81; positive predictive value, 0.58; and negative predictive value, 0.83.21
PET is indicated in patients with no clinical abnormalities and no signs of metastatic spread on CT who are cand idates for treatment with curative intent. It is useful for evaluating metastatic spread, except that occurring in the brain. PET is not required in patients with ground-glass opacities or clinical stage IA tumors with no other abnormality on chest CT. Regarding mediastinal staging, in a review of 4,105 patients with a median prevalence of nodal disease of 28%, staging values were as follows: sensitivity, 0.8; specificity, 0.88; positive predictive value, 0.75; and negative predictive value, 0.91.21 PET should provide the following information:
- Presence of normal or abnormal uptake in the primary tumor and quantification by maximum stand ardized uptake value (SUVmax)
- Presence of normal or abnormal uptake in hilar and mediastinal nodes and quantification by SUVmax
- Presence of normal or abnormal uptake in other parts of the lungs or in the rest of the body
Although SUVmax is subject to many intra- and interinstitutional variations, it is important to record it at initial staging to assess metabolic tumor response after treatment, especially after induction treatment to evaluate the possibility of tumor resection. SUVmax also has shown prognostic value, at least for Stage I-III squamous cell carcinomas and adenocarcinomas.22
Because PET has a poor anatomic resolution, the superimposition of PET with CT (e.g., with hybrid PET/CT scanners) may help the clinician locate the lesions with abnormal uptake. However, the mean staging values of combined PET/CT are similar to those of PET alone. In a review of 2,014 patients with a median prevalence of mediastinal nodal disease of 22%, the staging values for combined PET/CT were as follows: sensitivity, 0.62; specificity, 0.9; positive predictive value, 0.63; and negative predictive value, 0.9.21
The positive predictive value of PET is relatively low; therefore, histopathological confirmation of the lesions is recommended if this will affect therapy. Inflammations, granulomas, and infections may have high SUVmax, and if the correct histology remains unconfirmed, the patient may be excluded from radical treatment. If PET is not available, bone scanning and abdominal CT should be done to rule out metastatic spread.21
Magnetic resonance (MR) imaging has very specific indications in lung cancer staging. MR imaging of the brain currently is indicated in patients with Stage III and IV tumors, even if they have a negative clinical evaluation.21 It also is indicated in patients with brain metastasis identified on CT, as MR imaging may identify more lesions.23 It also may help define the involved anatomic structures in patients with apical (Pancoast) tumors or tumors invading the chest wall and mediastinum. MR imaging of the adrenals with in- and out-of-phase imaging may help exclude adrenal metastases in cases with indeterminate adrenal lesions on PET/CT.
The order in which the aforementioned anatomic and metabolic imaging tests are performed usually is chest X-rays first, followed by CT scan of the chest and upper abdomen, PET scan or PET/CT, and MR imaging in indicated cases.
The anatomic and metabolic imaging techniques described here provide a thorough description of the primary lesion and its local and distant spread, but do not provide its diagnosis. The TNM classification requires microscopic confirmation of malignancy24,25 and specification of histopathological type.14 The type of procedure used to obtain pathological confirmation of lung cancer differs depending on the location and spread of the tumor.
Sputum cytology may provide the diagnosis of lung cancer with high specificity. In a review of 29,145 patients, the diagnostic values of sputum cytology were as follows: sensitivity, 0.66; specificity, 0.99; false positive rate, 8%; and false negative rate, 10%.26 In certain patients with evident metastatic disease, this may be the only diagnostic test needed. However, molecular profiling of tumors is best performed on cell blocks; if these are not available in the sputum specimen, then larger samples may be needed.
Fiberoptic bronchoscopy is both a diagnostic and a staging procedure. As a diagnostic procedure including bronchial biopsy, brushings, washings, and endobronchial and transbronchial needle aspiration, its sensitivity is 0.88 and 0.78 for central and peripheral tumors, respectively.26 As a staging procedure, it shows the endobronchial location of the tumor: T2 if the main brochus is involved, regardless of its distance to the carina, and T4 if the carina is involved. It may suggest nodal involvement if there is extrinsic compression of the bronchi. The lymph nodes may be punctured with fine needles, either blindly—the classic transbronchial needle aspiration procedure—or with the assistance of EBUS and fine-needle aspiration (EBUS-FNA) or EUS and FNA (EUS-FNA). Peripheral tumors that remain undiagnosed by fiberoptic bronchoscopy may be diagnosed by transthoracic needle aspiration or biopsy, with a sensitivity of 0.9, a specificity of 0.97, a false positive rate of 1%, and a false negative rate of 22%.26
Thoracentesis and cytopathologic study of the pleural fluid may be enough in patients with malignant pleural effusion. It provides a diagnosis in 72% of patients.26 If cytology is negative, further pleural explorations with closed pleural biopsy and thoracoscopy should follow. Sensitivity and negative predictive values are both around 80% for closed pleural biopsy and greater than 80% and approximately 100%, respectively, for thoracoscopic biopsy.26 A malignant pleural effusion or tumor nodules on the pleural surface (parietal or visceral) classify the tumor as M1a. Thoracoscopy has the advantage of allowing exploration of the pleural cavity, lung surface, and mediastinum. Video-assisted thoracoscopic surgery also allows resection of peripheral nodules and assists in their diagnosis and staging. Ipsilateral hilar and mediastinal nodes may be biopsied as well.
The American College of Chest Physicians (ACCP) and the European Society of Thoracic Surgeons (ESTS) published guidelines on the preoperative staging of mediastinal lymph nodes.21,27 The 2013 ACCP Evidence-based Clinical Practice Guidelines favor invasive staging by needle aspiration techniques (EBUS-FNA, EUS-FNA) as the first procedures, but recommend confirmation with surgical biopsies (mediastinoscopy) if needle techniques are negative. In the absence of metastatic disease, the indications for invasive staging are as follows21:
- Discrete mediastinal lymph node enlargement with or without PET uptake in mediastinal lymph nodes
- PET activity in mediastinal lymph nodes and abnormal lymph nodes on CT
- High suspicion of N2 or N3 disease, either by lymph node enlargement on CT or PET uptake
- Intermediate suspicion of N2 or N3 disease by CT and PET and a central tumor or N1 disease
Invasive staging is not indicated for patients with extensive mediastinal infiltration or stage IA tumors with no suspicion of mediastinal lymph node involvement on CT or PET.21
The ESTS guidelines also recommend performing EBUS-FNA and EUS-FNA as the initial exploration in the following situations27:
- Positive mediastinal nodes on CT and /or PET or PET/CT
- Cases in which there is no evidence of N2-N3, but there is suspicion of N1 disease; central tumors larger than 3 cm; and adenocarcinomas with high PET uptake
Invasive staging may be avoided in patients with no evidence of mediastinal disease on CT and PET and tumors less than 3 cm in greatest dimension located peripherally, that is, in the outer third of the lung.
If needle techniques produce negative results, video-assisted mediastinoscopy is recommended to confirm the results or to identify mediastinal disease. In general, the negative predictive values of EBUS-FNA and EUS-FNA are too low, both in patients with normal and those with abnormal mediastinal lymph nodes, to make therapeutic decisions without proper confirmation by a surgical technique. In a recent article on the staging value of EBUS-FNA in patients with no mediastinal abnormalities, the sensitivity and negative predictive values for EBUS-FNA were 0.38 and 0.81, respectively, whereas they were 0.73 and 0.91 for mediastinoscopy.28 This article clearly highlights the importance of confirming negative results of EBUS-FNA and EUS-FNS with mediastinoscopy.
Additionally, the ESTS guidelines recommend exploration of the aortopulmonary window for left lung cancers and establish minimum requirements for mediastinoscopy in clinical practice: at least the inferior right and left paratracheal lymph nodes and the subcarinal lymph nodesshould be biopsied or removed; the superior right and left paratracheal lymph nodes and the hilar lymph nodes should be explored if there is evidence of involvement on CT or PET.27
Other invasive procedures should be performed as required, including pericardiocentesis or pericardioscopy, either transpleural or subxiphoid, for pericardial effusion; needle biopsies of liver and adrenal lesions; endoscopies of the gastrointestinal tract in cases of digestive symptoms or bleeding; and biopsy or excision of skin lesions.
These procedures should be performed sequentially from the least to most invasive: first, to rule out metastatic disease if imaging suggests metastatic spread, as this will avoid more invasive procedures; next, to rule out supraclavicular nodal disease (N3) if there is anatomic or metabolic suspicion; and finally, to explore the mediastinum as indicated by the aforementioned guidelines.