C.2. How is open repair of thoracic aneurysms performed?
Answer:
Ascending and Arch Aortic Aneurysms
Position: The surgical approach is through a median sternotomy with the patient positioned supine.
Cannulation for CPB: The CPB circuit requires a venous cannula that provides blood to the CPB pump and an arterial cannula that provides blood from the CPB pump to the patient. For aortic surgery, the arterial cannula can reside in any large artery, including the femoral, axillary, innominate, or carotid artery. Direct aortic cannulation can also be performed away from the site of the aneurysm if the anatomy allows it. Arterial cannulation can be accomplished either directly or with the use of a graft anastomosis to the target artery. Direct aortic and femoral cannulations are used less frequently because they carry a risk of embolization or malperfusion in patients with aortic dissection or atherosclerosis. Commonly, the axillary or innominate arteries are used for cannulation sites because they can provide adequate arterial flow during CPB and antegrade cerebral perfusion (ACP) during DHCA. Cannulation of these smaller arteries is achieved with end-to-side anastomosis with a synthetic graft. The venous cannula for CPB is commonly inserted into the right atrium or femoral vein.
Hypothermic circulatory arrest: Neurologic injury remains a major cause of morbidity and mortality after open aortic arch repair. Cerebral protection depends on decreasing metabolic demand and providing metabolic supply with adequate cerebral perfusion while excluding blood flow through the great vessels. Although DHCA improves ischemic tolerance of the brain considerably by suppressing metabolic demand, a major disadvantage of this technique is a limited "safe" time period of arrest before the risk of neurologic injury rises. Some authors have suggested that the "safe" time period of DHCA spans 45 to 60 minutes at 18 °C, while others have shown an increased risk of neurologic deficits with DHCA times that exceed 20 to 30 minutes. The incidence of postoperative stroke increases with greater than 40 minutes of DHCA. Another disadvantage of DHCA is the prolonged time needed to cool and rewarm patients on CPB, which predisposes patients to coagulopathy, systematic inflammatory response, organ ischemia reperfusion injury, or secondary cerebral damage from the release of free radicals. Moderate hypothermic circulatory arrest (MHCA) at 20 °C to 28 °C has been advocated for in recent years over DHCA, as it reduces bypass and cross-clamp times, efficiently prevents permanent neurologic deficits, and may be associated with decreased early in-hospital mortality relative to DHCA. Furthermore, MHCA provides comparable or superior visceral organ protection as it is associated with a lower incidence of renal failure when hypothermic circulatory arrest (HCA) was less than 30 minutes, whereas the risk is commensurate when HCA is greater than 30 minutes.
Adjunctive retrograde cerebral perfusion (RCP) and ACP techniques have been introduced to extend the "safe" time period of HCA, though the optimal temperature and duration of safe cerebral perfusion during arrest for arch reconstructive surgery still remain debatable. Direct delivery of oxygenated blood to the brain via ACP, which can be unilateral or bilateral, can be accomplished through cannulation of the brachiocephalic artery, the right axillary artery with snaring of the brachiocephalic artery to provide perfusion of the right common carotid artery, or the carotid artery; cannulation can increase the risk of thromboembolic events and air embolism. Unilateral and bilateral ACP permits tolerance of less than 50 minutes and up to 160 minutes of DHCA, respectively. Cerebral delivery of oxygenated blood through the venous system via RCP can be achieved by SVC cannulation with snaring above the right atrium. RCP allows for uniform cerebral cooling, decreased gaseous or particulate embolic events, flushing of accumulated toxins to limit acidosis, and deairing of arch vessels, though there is a potential increased risk of neurologic dysfunction. Outcomes data are inconclusive, and numerous variables affect the quality of cerebral protection, including flow rate, temperature, perfusion site(s), use of unilateral or bilateral perfusion, variable circle of Willis anatomy, embolization risk, and trauma to cranial vessels. RCP may be more likely utilized during short periods of HCA while bilateral ACP may be selected when longer periods are anticipated. There is no definitive recommendation for one method of cerebral protection and the choice of technique appears to be dictated by regional preferences as well as institutional practices.
Other neuroprotective strategies lack high-quality evidence to support their routine use. Topical head cooling is used to supplement cold perfusion to promote and sustain deeper hypothermia as well as to prevent rapid rewarming during emersion from HCA; concerns about this strategy include pressure on the eyes from the application of ice as well as interference with monitors such as electroencephalogram (EEG) and cerebral saturation probes. Barbiturates provide modest neuroprotection, but their true efficacy has been questioned. Corticosteroids have not been shown to be beneficial in stroke or in cardiac surgery. The antiapoptotic, osmotic, and free radical scavenging effects of mannitol are favorable, but no neuroprotective effects have been ascertained. Lidocaine and nimodipine are not associated with neuroprotection, although magnesium sulfate was associated with a lower incidence of new postoperative neurologic deficit.
Thoracoabdominal Aneurysms
Open TAAA repair remains the gold standard surgical approach, with long-term outcomes dependent on balancing the need for extensive aortic replacement with modulation of ischemia-related risk to the spinal cord and visceral organs.
Positioning: The surgical approach is typically through the left chest, with the patient positioned in a partial right lateral decubitus position, with the shoulders at 60° and the hips at 30°. The patient is supported with the use of a bean bag and an axillary roll placed under the patient's right axilla to minimize the chances of developing a brachial plexopathy.
Incision: A thoracoabdominal incision is the preferred approach for the majority of aneurysms. The proximal extent of the incision is dictated by the patient's body habitus and the aortic pathology. Typically, if the distal aortic arch and proximal descending aorta are to be exposed (Crawford types I and II), an incision to the fifth intercostal space is necessary. If the proximal extent of the aneurysm involves the distal thoracic aorta (Crawford types III and V), an incision to the 7th or 8th intercostal space is required. If the aneurysm is localized to the abdominal aorta (Crawford type IV), the proximal incision is made at the 9th or 10th intercostal space. The abdominal portion of the incision is typically limited to the retroperitoneum. Diaphragm preservation, defined as division only of the muscular portion of the diaphragm with preservation of the central tendinous portion, is performed to help improve postoperative respiratory mechanics.
Cannulation for and initiation of left-heart bypass: Open TAAA repair requires a period of ischemia to the lower portion of the body, including the spinal cord, viscera, and kidneys, while the aorta is cross-clamped. Left heart bypass (LHB) is utilized for patients with Crawford extent II aneurysms, extent I aneurysms due to dissection, and acute type B dissections. LHB provides isothermic oxygenated blood to the distal aorta to reduce the risk of spinal cord and visceral organ ischemia, permit longer cross-clamp times to complete the proximal anastomosis, and mitigate the acidosis that ensues after cross-clamp release. The pericardium is reflected or opened near the pulmonary veins, away from the phrenic nerve. The left atrium or pulmonary vein is cannulated, and blood is directed to the femoral artery via a centrifugal pump (Figure 9.16). Alternatively, passive axillary artery to femoral artery bypass can be used, which is dependent on the mean arterial blood pressure (MAP) proximal to the cross-clamp. In addition to the benefit of distal aortic perfusion, atrial-femoral bypass reduces preload to the left ventricle, reducing myocardial stress during cross-clamping. The use of LHB requires less systemic heparinization (100 units/kg given 5 minutes prior to cannulation) than CPB. Target activated clotting time (ACT) may vary from 200 to 300 seconds according to institutional preferences. Once LHB is initiated, the proximal aortic cross-clamp is applied to the aorta with careful attention to the preservation of the left recurrent laryngeal nerve. When possible, the proximal clamp is placed distal to the LSCA to preserve its contribution to spinal cord blood flow, though it may be positioned between the left common carotid artery and the LSCA if there is a large distal arch aneurysm. The distal clamp site is most commonly at the junction of the upper and middle thirds of the descending thoracic aorta anterior to the hemiazygos and intercostal veins. Once the aorta is clamped, the LHB flows are increased to a target of 1.5 to 2.5 L/min.
Figure 9.16.: Left Heart Bypass is Utilized for Distal Perfusion during Thoracoabdominal Aneurysm Repair.
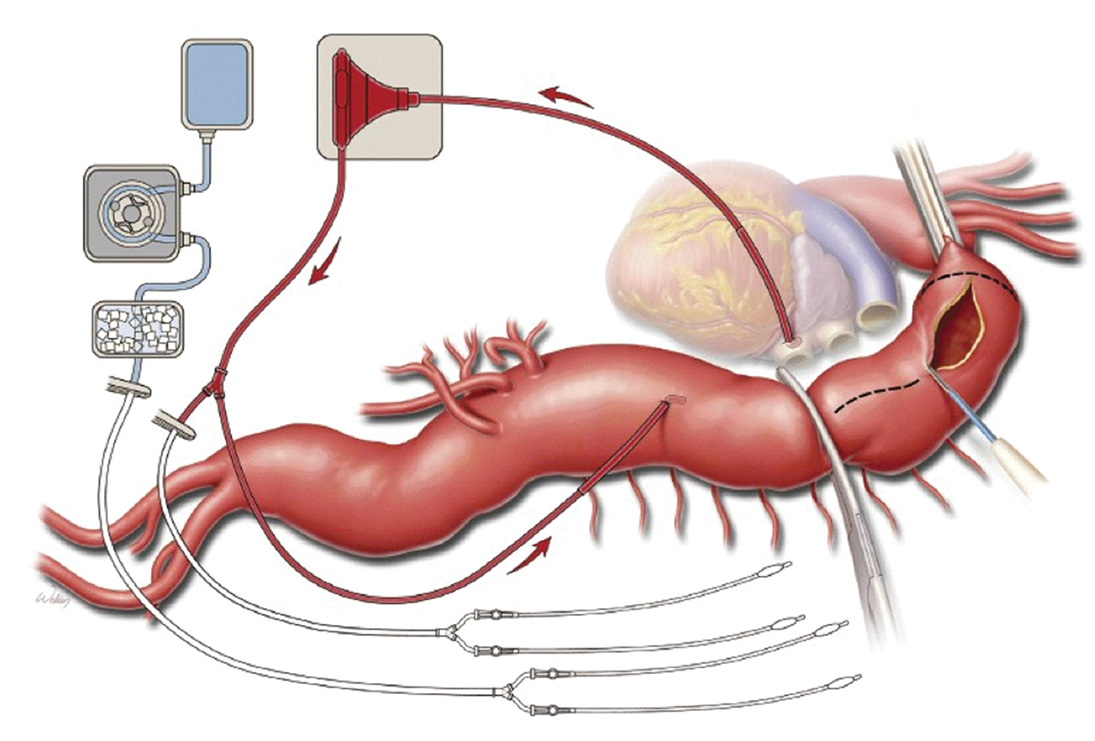
Left heart bypass is utilized for distal perfusion during thoracoabdominal aneurysm repair. The drainage cannula is placed in the left atrium, and the outflow cannula is placed in the thoracic aorta distal to the site of aortic cross-clamping. Additional catheters are available for selective perfusion of visceral arteries with blood during operative repair. A separate line may be set up to provide cold lactated Ringer solution through the renal arteries for renal protection. (Used with permission of AME Publishing Company, from de la Cruz KI, LeMaire SA, Weldon SA, et al. Thoracoabdominal aortic aneurysm repair with a branched graft. Ann Cardiothorac Surg. 2012;1:381-393; permission conveyed through Copyright Clearance Center, Inc.)
Construction of anastomoses and preservation of mesenteric and visceral organs: The proximal aorta is opened between the two cross-clamps, and the proximal anastomosis is constructed in an end-to-end fashion; when an elephant trunk is present, a graft-to-graft anastomosis is performed. Intercostal arteries in the T4 to T8 region are oversewn if they exhibit excessive retrograde bleeding indicative of good collateral flow to improve visualization, minimize hemorrhage, and to prevent a steal phenomenon of shunting blood away from the spinal cord. When feasible, patent intercostal and lumbar arteries between T7 and L2 are reattached to the graft if they have marginal back-bleeding to decrease the incidence of paralysis due to spinal cord ischemia. LHB is weaned and discontinued once the proximal anastomosis is complete. The distal clamp is removed to prepare the distal aorta for replacement and reapplied just distal to the intercostal anastomoses. Shed blood is collected by a cell saver system and rapidly auto-transfused back into the patient.
Visceral and renal perfusion is utilized to limit ischemic time. Selective visceral perfusion with isothermic blood is given at a rate of 500 mL/min using catheters inserted into the celiac axis and the superior mesenteric artery (SMA), which are connected to the LHB circuit. Cold renal perfusion with mannitol and methylprednisolone in crystalloid solution extending from a separate cooling circuit is delivered using catheters in the renal arteries approximately every 6 minutes at a rate of 300 mL/min for 1 to 2 minutes.
The configuration of visceral artery reimplantation is variable and determined by the age of the patient, the presence of connective tissue disease, the quality of the aortic tissue, and the distance between the ostia of the arteries. In older patients with degenerative aortic disease, the four arteries can be reattached as one patch if they are close to one another. Alternatively, each vessel can be reimplanted individually into a four-vessel branch graft in younger patients with connective tissue disease or those whose arterial origins are significantly separated. When using a multibranched graft, the distal aortic anastomosis can be completed prior to reattachment of the visceral branches in order to restore blood flow to the iliac arteries that provide collateral perfusion to the spinal cord. Depending on the degree of aneurysmal dilatation of the distal aorta, iliac, and femoral vessels, an end-to-end anastomosis can be sewn proximal to the bifurcation or distal to using a bifurcated abdominal aortic graft. Once the aortic cross-clamp is released after the completion of the distal aortic anastomosis, individual clamps remain on each of the four branches of the visceral graft while the right renal, SMA, celiac, and left renal artery anastomoses are completed, respectively. The inferior mesenteric artery is usually oversewn, but reimplantation may be necessary if the patient has bilateral hypogastric artery occlusions, previous colectomy, or a diseased SMA. Protamine and dye, such as indigo carmine or methylene blue, are subsequently administered to reverse the effect of heparin and assess the adequacy of renal perfusion.
Cannulation for CPB with or without HCA: If the proximal extent of TAAA aneurysm involves the arch, CPB and HCA ± ACP/RCP can be used in lieu of proximal aortic cross-clamping, which reduces the potential for spinal cord injury and preserves end-organ function. Typically, a venous cannula is placed via the femoral vein prior to or after the right lateral positioning of the patient. Arterial cannulation can reside in the distal aortic arch, proximal descending aorta, axillary artery, or femoral artery.
Clamp and sew technique: The "clamp and sew" technique involves cross-clamping the proximal aorta and replacing the aneurysmal aorta with a graft without the use of LHB or HCA. The technique poses a time limit to the surgeon as the patient's organs are not being supported during cross-clamp. This technique is typically inadequate for difficult repairs and is preferred for patients presenting with isolated descending thoracic aneurysms, aortic rupture, and hemodynamic instability.
Outcomes: Experienced centers have reported acceptable outcomes, such as an early mortality rate of less than 8% with a nadir of 5.6%. The largest published series of 3,309 open TAAA repairs observed an incidence of permanent paraplegia of 2.9%, paraparesis of 2.4%, postoperative stroke of 2.2%, chronic renal failure of 5.7%, and gastrointestinal ischemia of 0.9%. Octogenarians were not at higher risk for operative mortality or postoperative complications, although survival was significantly reduced relative to younger patients. Patients with heritable thoracic aortic disease and chronic aortic dissection (rather than degenerative aneurysms) had more favorable outcomes. Patients with preoperative renal failure or pulmonary disease as well as those who underwent emergent or complex Crawford extent II repairs were at higher risk for adverse events. Open TAAA repairs tend to be durable, such that 94% of patients did not require reinterventions at 15 years from their index procedures.
References
- Bilotta F, Gelb AW, Stazi E, Titi L, Paoloni FP, Rosa G. Pharmacological perioperative brain neuroprotection: a qualitative review of randomized clinical trials. Br J Anaesth. 2013;110:I113-I120.
- Cao L, Guo X, Jia Y, Yang L, Wang H, Yuan S. Effect of deep hypothermic circulatory arrest versus moderate hypothermic circulatory arrest in aortic arch surgery on postoperative renal function: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9:e017939.
- Di Marco L, Murana G, Leone A, Pacini D. Con-debate: short circulatory arrest times in arch reconstructive surgery: is simple retrograde cerebral perfusion or hypothermic circulatory arrest as good or better than complex antegrade cerebral perfusion for open distal involvement or hemi-arch? J Vis Surg. 2018;4:46.
- Fernandez Suarez FE, Fernandez Del Valle D, Gonzalez Alvarez A, Perez-Lozano B. Intraoperative care for aortic surgery using circulatory arrest. J Thorac Dis. 2017;9:S508-S520.
- Girardi LN, Lau C, Munjal M, et al. Impact of preoperative pulmonary function on outcomes after open repair of descending and thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg. 2017;153:S22-S29.e2.
- Girardi LN, Ohmes LB, Lau C, et al. Open repair of descending thoracic and thoracoabdominal aortic aneurysms in patients with preoperative renal failure. Eur J Cardiothorac Surg. 2017;51:971-977.
- Hong JC, Coselli JS. Open repair for thoracoabdominal aortic aneurysms precipitated by chronic aortic dissection. Vessel Plus. 2022;6:4.
- Kothari R, Weldon SA, Koksoy C, Coselli JS. Narrative review: open surgery for thoracoabdominal aortic aneurysm—is it still a horrible surgery? J Vis Surg. 2022;8:4.
- Malvindi PG, Scrascia G, Vitale N. Is unilateral antegrade cerebral perfusion equivalent to bilateral cerebral perfusion for patients undergoing aortic arch surgery? Interact Cardiovasc Thorac Surg. 2008;7:891-897.
- Ouzounian M, LeMaire SA, Weldon S, Coselli JS. Open repair of thoracoabdominal aortic aneurysm: step-by-step. Oper Tech Thorac Cardiovasc Surg. 2018;23:2-20.
- Reed H, Berg KB, Janelle GM. Aortic surgery and deep-hypothermic circulatory arrest: anesthetic update. Semin Cardiothorac Vasc Anesth. 2014;18:137-145.


