A.2. What are the major changes in the loading conditions of the LV that result from the four different lesions? Why do they occur? What changes result from them?
Answer:
AS represents a chronic systolic pressure load on the LV. This increases the wall tension (stress) in accordance with Laplace's law:
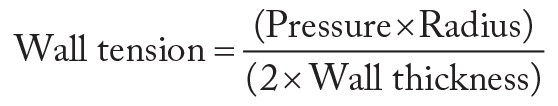
The LV undergoes parallel duplication of muscle fibers to compensate for increased wall stress. This results in increased wall thickness (concentric hypertrophy) and some decrease in radius, thereby normalizing wall stress. If the MV remains competent, then little change occurs in the other cardiac chambers.
AI causes left ventricular diastolic volume overload due to the additional volume flowing retrograde into the LV across the incompetent AV during diastole. This results in eccentric hypertrophy and left ventricular dilation (increased muscle mass and chamber radius). However, AI decreases aortic diastolic pressure, which is the pressure that must be exceeded by the contracting LV to open the AV and result in left ventricular systolic ejection. Therefore, left ventricular work is performed against a lower outflow impedance (aortic diastolic pressure). Stroke volume and ejection fraction may be preserved until late in the disease process. As with AS, the presence of a competent MV confines the changes to the LV. However, the left ventricular dilation that follows chronic AI may result in mitral annular dilation or alteration in the PM orientations with resultant MR. Left atrial enlargement secondary to MR, or left atrial pressure overload due to increasing left ventricular end-diastolic pressure (LVEDP) in the course of AI, can occur.
MS results in a chronically underfilled LV because of progressive obstruction to left atrial emptying. This chronic underloading condition can result in diminished contractile function ("disuse atrophy"). In addition, if the cause of the MS is rheumatic, then myofibril damage can also occur. Contrary to the left ventricular pressure and volume underloading, the LA is both pressure and volume overloaded to maintain flow across the progressively narrowing mitral orifice. According to Gorlin's equation for pressure gradient,
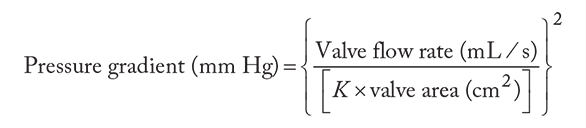
the pressure gradient increases by the square of any increase in flow rate or decrease in valve area. The elevated left atrial pressure leads to left atrial hypertrophy and eventually dilation that predisposes to premature atrial contractions and subsequent atrial fibrillation. The loss of atrial contraction will further diminish the flow across the stenotic MV. At the same time, elevated left atrial pressure hinders pulmonary venous flow and causes pulmonary vascular engorgement. This leads to intimal hypertrophy that induces irreversible elevations in pulmonary vascular resistance. As a consequence of pulmonary hypertension, the right ventricular (RV) systolic pressure increases and right ventricle dilates, potentially leading to RV failure and tricuspid regurgitation (TR).
MR results in volume overload of the LV. In MR, the LV ejects into both systemic circulation (across the AV; high afterload and low compliance) and retrograde to the LA (low afterload and high compliance) across the incompetent MV. This "back and forth" of the regurgitant volume results in left ventricular volume overload. However, this additional work is performed at a low pressure; therefore, left ventricular wall tension is minimally increased initially. As with AI, the volume overload results in marked left ventricular dilation and eccentric hypertrophy. At the same time, the LA is also volume overloaded and undergoes dilation. When the volume overload occurs slowly, the pulmonary pressures increase minimally despite large regurgitant volumes. In contrast, acute MR, as can occur following acute myocardial infarction with PM rupture, presents a sudden volume overload to the LA. Without time to dilate, the left atrial pressure rises rapidly, thereby limiting pulmonary drainage with resultant pulmonary engorgement and acute increase in PA pressures.
References
- Lilly LS. Valvular heart disease. In: Lilly LS, ed. Pathophysiology of Heart Disease: An Introduction to Cardiovascular Medicine. 7th ed. Wolters Kluwer; 2021:202-231.
- Ramakrishan H, Craner RC, Devaleria PA, Cook DJ, Housmans PR, Rehfeldt KH. Valvular heart disease: replacement and repair. In: Kaplan JA, Augustides J, Maneck GR, Maus T, eds. Kaplan's Cardiac Anesthesia. 7th ed. Elsevier; 2017:770-817.


