Acute General Rx
- Fig. 3 shows a treatment algorithm for STEMI. Assessment and treatment algorithm for non-ST-segment MI is described in Fig. 4. Rationale of the treatment of a patient with STEMI is based on “time is muscle.” Therefore all communities should create and maintain a regional system of STEMI care that includes assessment and continuous quality improvement of EMS and hospital-based activities. A 12-lead ECG must be done by EMS personnel at the site of first medical contact (FMC).
- Reperfusion therapy should be administered to all eligible patients with STEMI with symptom onset within 12 hr. Indications for primary angioplasty and comparison with fibrinolytic therapy are described in Table 3. Primary PCI is the recommended method of reperfusion when it can be performed in a timely fashion by experienced operators with an ideal FMC-to-device time system goal of 90 min or less.
- In the absence of contraindications, fibrinolytic therapy (Table 4) should be administered to patients with STEMI at non-PCI-capable hospitals when the anticipated FMC-to-device time at a PCI-capable hospital exceeds 120 min because of unavoidable delays. Door-in door-out time (DIDO) for fibrinolytic therapy should be less than 30 min. If more than 30 min delay, transfer the patient to a PCI-capable hospital.
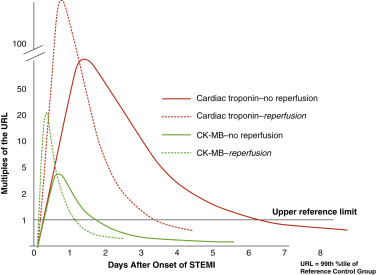
- Among STEMI patients who were treated with fibrinolytics, patients with >50% ST-segment resolution on EKG were at much lower risk for cardiac-related mortality compared with those with <50% resolution at 30 days.
- PCI is superior to thrombolytic therapy and is the standard of care. It is effective and generally results in more favorable outcomes than thrombolytic therapy.
- Primary PCI should be performed in patients with STEMI and persistent ischemic symptoms and who have contraindications to fibrinolytic therapy, irrespective of the time delay from FMC, or in patients with cardiogenic shock or acute severe HF irrespective of time delay from myocardial infarction (MI) onset, first medical contact to balloon time is <90 min or door to balloon/door to needle time is <1 hr, symptoms onset was >3 hr ago and when diagnosis of STEMI in doubt. Coronary stents (drug-eluting or bare-metal) are useful in patients with STEMI (3,4,5 &6).
- The question of culprit vessel vs. complete revascularization during PCI has been brought up since the stent technology was applied to the management of STEMI. The most recent clinical trials (CuLPRIT, PRAMI, and DANAMI3-PRIMULTI [FFR-driven revascularization]) appear to favor complete revascularization in the setting of STEMI. However, CULPRIT-SHOCK trial showed culprit vessel only PCI associated with 9.5% absolute reduction in the rate of death or renal replacement therapy at 30 days compared to multivessel PCI in acute MI patients with cardiogenic shock. One-yr outcomes did not show significant difference in mortality between two groups. Korea Acute Myocardial Infarction-National Institutes of Health (KAMIR-NIH) Registry data showed better outcomes with multivessel PCI in cardiogenic shock patients compared to culprit vessel-only PCI. Thus multivessel PCI should be reserved for few selective patients.
- For patients presenting to a non-PCI-capable hospital, rapid assessment should be done of (1) the time from onset of symptoms, (2) the risk of complications related to STEMI, (3) the risk of bleeding with fibrinolysis, (4) the presence of shock or severe HF, and (5) the time required for transfer to a PCI-capable hospital and a decision about administration of fibrinolytic therapy reached. Because the effectiveness of thrombolytics is time dependent, these agents should ideally be administered either in the field or within 30 min of the patient’s arrival to the emergency department (door-to-needle time).
- Fibrinolytic therapy: If tissue plasminogen activator (t PA) or reteplase is used, anticoagulants, such as heparin, are given to increase the likelihood of patency in the infarct-related artery for 48 hr and preferably for the duration of the index hospitalization, up to 8 days. In patients receiving fibrinolysis for STEMI, treatment with enoxaparin is superior to treatment with unfractionated heparin for 48 hr but is associated with an increase in major bleeding episodes. In patients receiving streptokinase or APSAC, heparin after thrombolysis is not indicated because it does not offer any additional benefit and can result in increased bleeding complications. Tenecteplase and reteplase are comparable with accelerated infusion recombinant t-PA in terms of efficacy and safety but are more convenient because they are administered by bolus injection. Lanoplase and heparin bolus plus infusion are as effective as tPA with regard to mortality rate, but the rate of intracranial hemorrhage is significantly higher.
- Absolute contraindications to thrombolytic therapy (Table 5) include history of intracranial hemorrhage, known intracranial malignant neoplasm or arteriovenous malformation, ischemic stroke within 3 mo (except acute ischemic stroke within 4.5 hr), suspected aortic dissection, active bleeding or bleeding diathesis (except menses), significant closed head or facial trauma within 3 mo, intracranial or intraspinal surgery within 2 mo, or severe uncontrolled hypertension (unresponsive to therapy). For streptokinase, this applies to prior treatment within 6 mo.
- Relative contraindications: History of chronic severe, poorly controlled hypertension, SBP >180 mm Hg, diastolic blood pressure (DBP) >110 mm Hg, history of prior ischemic stroke more than 3 mo, dementia, known intracranial pathology, traumatic or prolonged cardiopulmonary resuscitation (CPR) (>10 min), major surgery <3 wk, recent internal bleeding within 2 to 4 wk, noncompressible vascular punctures, pregnancy, active peptic ulcer, oral anticoagulant therapy. After the administration of thrombolytics, immediate transfer to a PCI-capable facility is advisable without waiting for lytic results.
- Transfer to a PCI-capable hospital: Immediate transfer for STEMI patients who develop cardiogenic shock or acute severe HF, irrespective of the time delay from MI onset. Urgent transfer if the patient demonstrates evidence of failed reperfusion or reocclusion after fibrinolytic therapy.
- Coronary angiography should not be performed within the first 2 to 3 hr after administration of fibrinolytic therapy.
- Coronary artery bypass graft (CABG): Urgent CABG is indicated in patients with STEMI and coronary anatomy not amenable to PCI who have ongoing or recurrent ischemia, cardiogenic shock, severe HF, or other high-risk features. CABG is recommended in patients with STEMI at time of operative repair of mechanical defects. Box 1 summarizes CABG in patients with MI.
- Therapeutic hypothermia should be started as soon as possible in comatose patients with STEMI and out-of-hospital cardiac arrest caused by ventricular fibrillation (VF) or pulseless ventricular tachycardia, including patients who undergo primary PCI.
- Immediate angiography and PCI when indicated should be performed in resuscitated out-of-hospital patients.
- The use of mechanical circulatory support is reasonable in patients with STEMI who are hemodynamically unstable and require urgent CABG.
- For NSTEMI patients, immediate invasive strategy (within 2 hr) recommended in patients with refractory angina, signs or symptoms of congestive heart failure or new or worsening ischemic mitral regurgitation, hemodynamic instability, recurrent angina or ischemia at rest or with low level activities despite intensive medical therapy and sustained ventricular tachycardia or ventricular fibrillation.
- Early invasive strategy (<24 hr) for NSTEMI patients recommended if GRACE risk for more than 140, dynamic ST changes on EKG and temporal change in troponin levels.
- NSTEMI patient with low-risk TIMI score (0 or 1) and/or low GRACE score (<109) and/or troponin-negative female patients can benefit from ischemia-guided strategy. Fibrinolytic therapy is contraindicated in NSTEMI patients.
- Medical therapy should be initiated immediately in the emergency department for all MI patients. This includes:
- Routine measures
- Oxygen: Supplemental oxygen should be administered to patients with arterial oxygen desaturation (SaO2 less than 90%). No benefit has been demonstrated to supplemental oxygen in patients with normal SaO2.
- Nitroglycerin: Increase oxygen supply by reducing coronary vasospasm and decrease oxygen consumption by reducing ventricular preload. Patients with ongoing ischemic discomfort should receive sublingual nitroglycerin every 5 min for a total of three doses, after which an assessment should be made about the need for intravenous nitroglycerin. Intravenous nitroglycerin is indicated for relief of ongoing ischemic discomfort, control of hypertension, or management of pulmonary congestion. Nitrates should not be administered to patients whose systolic blood pressure is <90 mm Hg or ≥30 mm Hg below baseline or severe bradycardia (<50 beats/min), tachycardia (>100 beats/min), or suspected RV infarction. Nitrates should not be administered to patients who have received a phosphodiesterase inhibitor for erectile dysfunction within the last 24 hr (48 hr for tadalafil).
- Adequate analgesia: Morphine sulfate 2 to 4 mg intravenous (IV) initially with increments of 2 to 8 mg IV at 5- to 10-min intervals can be given for severe pain unrelieved by nitroglycerin. Morphine can reduce the catecholamine surge caused by anxiety and pain, particularly in patients with anterior myocardial infarctions, which in turn can reduce heart rate and pulmonary capillary wedge pressure (PCWP), the increased cardiac workload and oxygen demand, leading to decreased ischemia and pulmonary congestion. Hypotension from morphine can be treated with careful IV hydration with saline solution. If sinus bradycardia accompanies hypotension, use atropine (0.5 to 1.0 mg IV q5min prn to a total dose of 2.5 mg). Respiratory depression caused by morphine can be reversed with naloxone 0.8 mg. Morphine sulfate and nitroglycerine should be avoided in patients with RV involvement who usually present with bradycardia and hypotension. Pain management in these cases should be provided preferentially with meperidine 25 to 50 mg intravenously q4h, in combination with Phenergan 12.5 mg to prevent nausea and/or vomiting. Blood pressure support with normal saline solution is of critical importance to maintain adequate hemodynamics until optimal revascularization is accomplished.
- Aspirin 162 to 325 mg PO should be crushed and chewed to enhance drug absorption and delivery. It should be given as soon as possible and continued indefinitely at 81 mg daily. Depending on the clinical and ECG findings, if the patient is suspected to have a coronary anatomy that needs CABG rather than PCI, aspirin should be continued. P2Y12 receptor antagonists should be avoided (except cangrelor) because they increase the perioperative bleeding risk; on-pump surgery should be deferred for at least 24 hr after clopidogrel and ticagrelor. Off-pump surgery might be considered within 24 hr of clopidogrel or ticagrelor if the benefits of revascularization outweigh the risk of bleeding. However, if the coronary artery disease is likely to benefit from PCI alone, then a loading dose of clopidogrel 600 or 300 mg or ticagrelor 180 mg PO or prasugrel 60 mg should be given as early as possible and no later than 1 hr after PCI. P2Y12 receptor antagonist should be continued for at least 1 yr after acute coronary syndrome or after primary PCI. Prasugrel showed significant net clinical benefit (MACE vs. bleeding complications) only in patients with MI who underwent revascularization. It shouldn’t be given for non-revascularized patients. Ticagrelor or clopidogrel can be given in patients with MI with or without catheter-based revascularization. Cangrelor is the newest direct-acting P2Y12 platelet receptor inhibitor. It has a similar chemical structure to ATP, with a half-life of 3 to 6 min. It is given IV as a bolus plus 120 min of infusion at the time of primary PCI in patients who are naïve to P2Y12 receptor antagonists. It was approved by the FDA in 2015 after the CHAMPION PHOENIX trial. Clopidogrel and prasugrel should be started after its infusion is finished. The ticagrelor loading dose can be given during the infusion. Considering rapid onset action and clearance, cangrelor can be started in the emergency room at the time of high-risk acute myocardial infarction diagnosis irrespective surgical or catheter-based revascularization.
- In patients receiving fibrinolytics only or balloon angioplasty without stent, P2Y12 antagonists can be given for as little as 14 days. Clopidogrel is recommended for postfibrinolytic patients.
- Unfractionated heparin (UFH) is recommended in all patients with NSTEMI and STEMI (fibrinolysis or invasive revascularization). UFH infusion should not exceed more than 48 hr after PCI or fibrinolysis in the absence of an ongoing indication due to risk of heparin-induced thrombocytopenia. NSTEMI patients who underwent ischemia-guided therapy, low-molecular- weight heparin (LMWH) showed better MACE outcomes compared with UFH. The benefit was not significant in revascularized patients. Bivalirudin was associated with lower MACE and bleeding events in STEMI patients compared with UFH. However, it increased the risk of stent thrombosis. In NSTEMI patients who are undergoing PCI, LMWH, bivalirudin, and UFH are acceptable.
- Beta-adrenergic blocking agents should generally be given to all patients who do not exhibit evidence of shock. Table 6 summarizes recommendations for β-blocker therapy for STEMI. β-blockers are useful to reduce myocardial oxygen consumption and prevent tachyarrhythmias. Early IV beta blockage (in the initial 24 hr) followed by institution of an oral maintenance regimen is also effective in reducing recurrent infarction and ischemia. Oral β-blockers should be initiated in the first 24 hr in patients with MI who do not have any of the following: Signs of HF, evidence of a low-output state, sinus tachycardia, increased risk for cardiogenic shock, or other contraindications for its use (bradycardia, PR interval more than 0.24 sec, second- or third-degree heart block, active asthma, or reactive airways disease).
- They should be continued during and after hospitalization for all patients with MI and with no contraindications to their use for at least 2 yr. Patients with initial contraindications to the use of β-blockers in the first 24 hr after MI should be reevaluated to determine their subsequent eligibility. It is reasonable to administer intravenous β-blockers at the time of presentation to patients with MI and no contraindications to their use who are hypertensive or have ongoing ischemia.
- In patients with acute MI, treatment with drug-eluting stents is associated with decreased mortality rates and a reduction in the need for repeated revascularization procedures compared with treatment including bare-metal stents.
- Gp IIb/IIIa inhibitors in the era of DAPT therapy and primary PCI have failed to show benefit with “upstream” treatment. Abciximab might be useful in the presence of large thrombus burden during primary PCI. For patients receiving bivalirudin as the primary anticoagulant, routine adjunctive use of GP IIb/IIIa inhibitors is not recommended but may be considered as adjunctive or “bail-out” therapy in selected cases. In patients with acute coronary syndrome with high-risk features and not adequately pretreated with P2Y12 inhibitors, it is useful to administer GP IIb/IIIa inhibitors at the time of PCI.
TABLE 6 Recommendations for β-Blocker Therapy for ST-Elevation Myocardial Infarction (STEMI)
| Recommendation | COR | LOE |
|---|
Oral β-blockers should be initiated in the first 24 hr in patients with STEMI who do not have any of the following:
Signs of heart failure or evidence of a low-output state
Increased risk for cardiogenic shock∗:
- Age >70 yr
- Systolic blood pressure <120 mm Hg
- Sinus tachycardia >110 beats/min or heart rate <60 beats/min
- Increased time since the onset of symptoms of STEMI
Other relative contraindications to use of oral β-blockers:
- PR interval longer than 0.24 sec
- Second- or third-degree heart block
- Active asthma or reactive airways disease
| I | B |
| β-blockers should be continued during and after hospitalization for all patients with STEMI and no contraindications to their use. | I | B |
| Patients with initial contraindications to the use of β-blockers in the first 24 hr after STEMI should be reevaluated to determine their subsequent eligibility. | I | C |
| It is reasonable to administer IV β-blockers at initial encounter to patients with STEMI and no contraindications to their use who are hypertensive or have ongoing ischemia. | IIa | B |
COR, Class of recommendation; IV, intravenous; LOE, level of evidence.
Modified from O’Gara PT et al: 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, J Am Coll Cardiol 61:e78, 2013. In Zipes DP: Braunwald’s heart disease, a textbook of cardiovascular medicine, ed 11, Philadelphia, 2019, Elsevier.
BOX 1 CABG in Patients With Acute MI
Class I- Emergency CABG is recommended in patients with acute MI in whom (1) primary PCI has failed or cannot be performed, (2) coronary anatomy is suitable for CABG, and (3) persistent ischemia of a significant area of myocardium at rest or hemodynamic instability refractory to nonsurgical therapy is present.
- Emergency CABG is recommended in patients undergoing surgical repair of a postinfarction mechanical complication of MI, such as ventricular septal rupture, mitral valve insufficiency because of papillary muscle infarction or rupture, or free wall rupture.
- Emergency CABG is recommended in patients with cardiogenic shock and who are suitable for CABG irrespective of the time interval from MI to onset of shock and time from MI to CABG.
- Emergency CABG is recommended in patients with life-threatening ventricular arrhythmias (believed ischemic in origin) in the presence of left main stenosis greater than or equal to 50% or three-vessel CAD.
Class IIa- The use of CABG is reasonable as a revascularization strategy in patients with multivessel CAD with recurrent angina or MI within the first 48 hr of STEMI presentation as an alternative to a more delayed strategy.
- Early revascularization with PCI or CABG is reasonable for selected patients older than 75 yr of age with ST-segment elevation or left bundle branch block who are suitable for revascularization irrespective of the time interval from MI to onset of shock.
Class III- Emergency CABG should not be performed in patients with persistent angina and a small area of viable myocardium who are stable hemodynamically.
- Emergency CABG should not be performed in patients with no reflow (successful epicardial reperfusion with unsuccessful microvascular reperfusion).
|
From Hillis LD et al: 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, J Am Coll Cardiol 58:e123-e210, 2011. In Parrillo JE et al: Critical care medicine, principles of diagnosis and management in the adult, ed 5, Philadelphia, 2019, Elsevier.
TABLE 5 Contraindications to and Cautions in the Use of Fibrinolytics for Treating ST-Elevation Myocardial Infarction∗
| Absolute Contraindications |
Any previous intracranial hemorrhage
Known structural cerebral vascular lesion (e.g., arteriovenous malformation)
Known malignant intracranial neoplasm (primary or metastatic)
Ischemic stroke within 3 mo except acute ischemic stroke within 4.5 hr
Suspected aortic dissection
Active bleeding or bleeding diathesis (excluding menses)
Significant closed-head or facial trauma within 3 mo
Intracranial or intraspinal surgery within 2 mo
Severe uncontrolled hypertension (unresponsive to emergency therapy)
For streptokinase, previous treatment within the previous 6 mo |
| Relative Contraindications |
History of chronic, severe, poorly controlled hypertension
Significant hypertension at initial evaluation (SBP >180 mm Hg or DBP >110 mm Hg)†
History of previous ischemic stroke >3 mo
Dementia
Known intracranial pathology not covered in Absolute Contraindications
Traumatic or prolonged (>10 min) cardiopulmonary resuscitation
Major surgery (<3 wk)
Recent (within 2 to 4 wk) internal bleeding
Noncompressible vascular punctures
Pregnancy
Active peptic ulcer
Oral anticoagulant therapy |
DBP, Diastolic blood pressure; MI, myocardial infarction; SBP, systolic blood pressure.
From O’Gara PT et al: 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, J Am Coll Cardiol 61:e78, 2013. In Zipes DP: Braunwald’s heart disease, a textbook of cardiovascular medicine, ed 11, Philadelphia, 2019, Elsevier.
TABLE 4 Dosing Regimens of Commonly Used Thrombolytic Agents
| Thrombolytic Agents | Dosing Regimen |
|---|
| t-PA (alteplase) | 15 mg bolus IV, followed by 0.75 mg/kg body weight (not to exceed 50 mg) over 30 min, followed by 0.5 mg/kg (not to exceed 35 mg) over 60 min |
| r-PA (reteplase) | Two 10-U IV boluses, given 30 min apart |
| TNK-t-PA (tenecteplase) | Single bolus IV 0.5 mg/kg (dose rounded to the nearest 5 mg, ranging from 30 to 50 mg) |
| Streptokinase | 1.5 million U IV over 60 min |
IV, Intravenous; PA, plasminogen activator; r-PA, recombinant plasminogen activator; TNK-t-PA, tenecteplase tissue plasminogen activator; t-PA, tissue plasminogen activator; U, units.
From Andreoli TE et al: Andreoli and Carpenter’s Cecil essentials of medicine, ed 8, Philadelphia, 2010, Saunders.
TABLE 3 Indications for Primary Angioplasty and Comparison With Fibrinolytic Therapy
| Indications |
| Alternative recanalization strategy for ST segment elevation or LBBB acute MI within 12 hr of symptom onset (or >12 hr if symptoms persist) |
| Cardiogenic shock developing within 36 hr of ST segment elevation/Q wave acute MI or LBBB acute MI in patients >75 yr old who can be revascularized within 18 hr of shock onset |
| Recommended only at centers performing >200 PCI/yr with backup cardiac surgery and for operators performing <75 PCI/yr |
| Advantages of Primary PCI |
| Higher initial recanalization rates |
| Reduced risk of intracerebral hemorrhage |
| Less residual stenosis; less recurrent ischemia or infarction |
| Usefulness when fibrinolysis contraindicated |
| Improvement in outcomes with cardiogenic shock |
| Disadvantages of Primary PCI (Compared With Fibrinolytic Therapy) |
| Access, advantages restricted to high-volume centers, operators |
| Longer average time to treatment |
| Greater dependence on operators for results |
| Higher system complexity, costs |
LBBB, Left bundle branch block; MI, myocardial infarction; PCI, percutaneous coronary intervention (includes balloon angioplasty, stenting).
From Goldman L, Schafer AI: Goldman’s Cecil medicine, ed 24, Philadelphia, 2012, Saunders.
Figure 3 Reperfusion therapy for patients with STEMI.

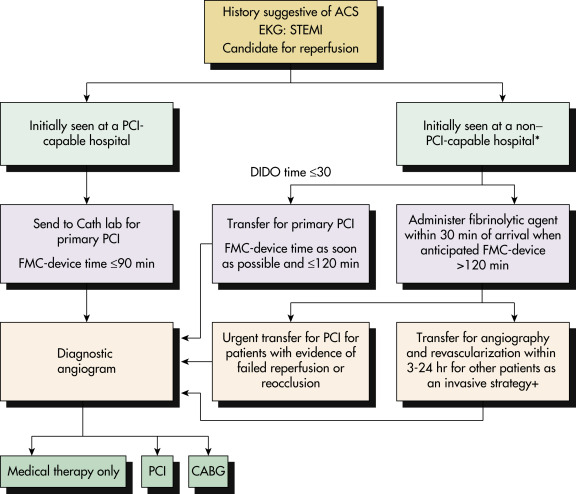
∗Patients with cardiogenic shock or severe heart failure initially seen at a non-PCI-capable hospital should be transferred for cardiac catheterization and revascularization as soon as possible, irrespective of time delay from MI onset. +Angiography and revascularization should not be performed within the first 2 to 3 hr after administration of fibrinolytic therapy. ACS, Acute coronary syndrome; CABG, coronary artery bypass graft; Cath, catheterization; DIDO, door-in to door-out; EKG, electrocardiogram; FMC, first medical contact; MI, myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction.
Modified from O’Gara PT et al: 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, JACC 61(4):e78-e140, 2013.
Figure 4 Algorithm for Management of Patients with Definite or Likely Non-ST-Elevation Acute Coronary Syndromes (Nste-ACS)
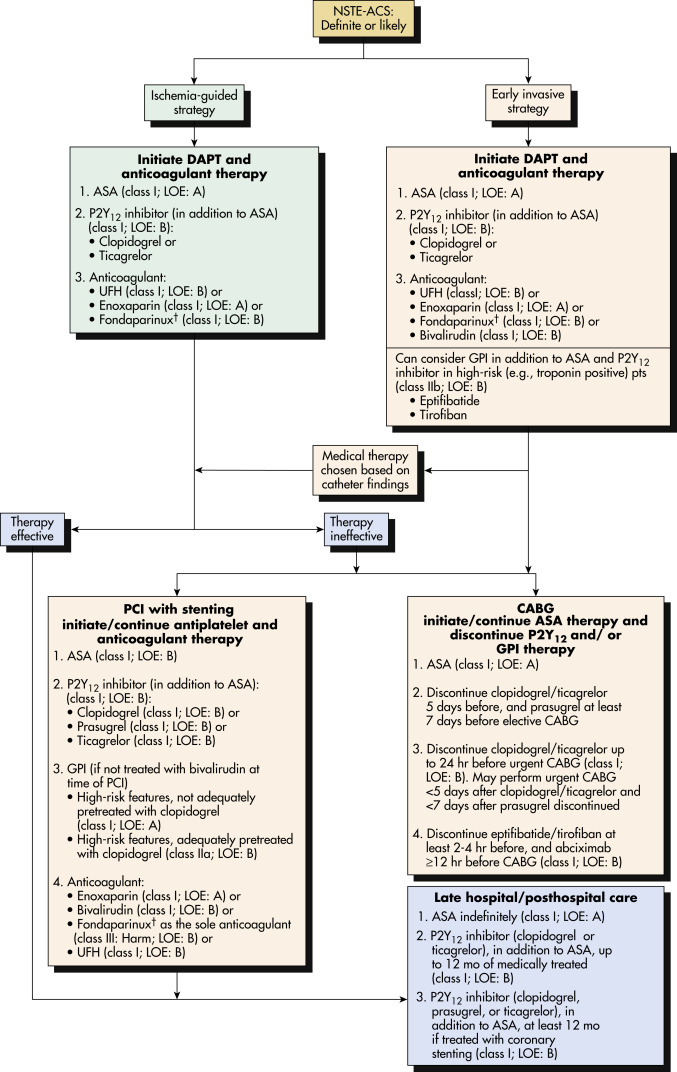
†In patients who have been treated with fondaparinux (as upfront therapy) who undergo percutaneous coronary intervention (PCI), an additional anticoagulant with anti-IIa activity should be administered at the time of PCI because of the risk of catheter thrombosis. ASA,Acetylsalicylic acid (aspirin); CABG, coronary artery bypass grafting; DAPT, dual-antiplatelet therapy; GPI, glycoprotein inhibitor; LOE, level of evidence; pts, patients; UFH, unfractionated heparin.
From Amsterdam EA et al: 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, J Am Coll Cardiol 64:e139-228, 2014. In Zipes DP: Braunwald’s heart disease, a textbook of cardiovascular medicine, ed 11, Philadelphia, 2019, Elsevier.
Chronic Rx
- Discharge medications in all patients with MI (unless contraindicated) should include antiischemic medications (e.g., nitroglycerin, β-blocker), lipid-lowering agents, and antiplatelet therapy (aspirin and/or P2Y12 antagonists).
- Aspirin, 81 mg PO daily, but should be continued indefinitely unless not tolerated (e.g., GI bleed). In MI patients, clopidogrel 75 mg PO daily; ticagrelor, 90 mg bid, or prasugrel, 10 mg PO daily, can be combined with aspirin and should be continued without interruption for a minimum of 12 mo after drug-eluting stent placement; however, aspirin should be continued indefinitely. In cases of high bleeding risk or significant overt bleeding, consider discontinuation of P2Y12 inhibitor after 6 mo (Fig. 5). Combining P2Y12 antagonists with aspirin reduces risk for repeat myocardial infarction and stent thrombosis. If there is an elective surgical intervention pending, it is recommended to defer the surgery until completion of the full course of the P2Y12 antagonist treatment. Duration dual antiplatelet therapy in high-bleeding-risk (HBR) patients is debatable. Many randomized controlled trials support single antiplatelet therapy 1 or 3 mo after PCI in HBR acute coronary syndrome populations.
- Angiotensin-converting enzyme inhibitors (ACEIs) should be started within the first 24 hr of MI to all patients having MI with anterior infarction, pulmonary congestion, or LV EF <40%, in the absence of hypotension. They reduce LV dysfunction and dilation and slow the progression to HF during and after acute MI. Angiotensin receptor blockers (ARBs) should be given to patients who have indication but are intolerant of ACEIs. IV formulations of ACEIs should not be given within the first 24 hr of STEMI due to risk of hypotension. ARBs offer no advantage over ACEIs and should be considered only in patients who are intolerant to ACEIs.
- ACEIs may be stopped in patients without complications and no evidence of LV dysfunction after 6 to 8 wk.
- ACEIs should be continued indefinitely in patients with impaired LV function (EF <40%) or clinical HF.
- Long-term aldosterone antagonist therapy should be prescribed for post-MI patients without significant renal dysfunction (creatinine ≤2.5 mg/dl in men and ≤2.0 mg/dl in women) or hyperkalemia who are already taking an ACEI, a β-blocker, and have LV EF <40% with symptomatic HF or diabetes.
- In late 2018, American College of Cardiology (ACC) recommended LDL goal of <70 mg/dl for secondary prevention of atherosclerotic cardiovascular disease. Goal should further be reduced to <55mg/dl in patients with existing atherosclerotic vascular disease and familial hypercholesterolemia. High-intensity statins (atorvastatin 40 to 80 mg or rosuvastatin 20 to 40 mg) should be started as early as possible in all patients with MI regardless of lipid panel, not only for their lipid-lowering effects, but also their antiinflammatory properties (JUPITER trial), which can stabilize the ruptured plaque. Atorvastatin 80 mg can be used (PROVE IT-TIMI 22 and MIRACL trials). IMPROVE-IT trial showed adding ezetimibe to statin treatment could decrease recurrent MI and ischemic stroke in MI patients. FDA also approved two PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors (alirocumab and evolocumab) for heterozygous familial hypercholesterolemia patients who are receiving maximally tolerated statins or patients with clinical atherosclerotic cardiovascular disease who require lowering LDL levels. Newer 2018 lipid guidelines suggested starting PCSK9 inhibitor in very high-risk atherosclerotic cardiovascular disease patients who did not meet the LDL goal on high intensity statin and ezetimibe. One should always consider adding ezetimibe to high intensity statin prior to initiation of PCSK9 inhibitor due to cost issues. A fasting lipid panel should be checked during the first 24 hr of hospital course, and the intensive therapy can be stepped down if appropriate. Inclisiran, evinacumab and bempedoic acid are reasonable non-statin therapy alternatives in patients who are unable to tolerate statin and reach target LDL levels.
- In diabetic patients, HbA1c goal should be aimed at below or around 7.0% to reduce micro- and macrovascular complications. Oral hypoglycemic agents GLP1 (glucagon-like peptide) agonist (liraglutide) and SGLT2 (sodium-glucose cotransporter) inhibitor (empagliflozin) showed significant mortality benefit in type 2 diabetic patients with history of CAD.
- ACC/AHA 2017 hypertension guidelines recommend initiation of blood pressure (BP)-lowering medications in patients with clinical CVD and an average SBP ≥130 mm Hg or a DBP ≥80 mm Hg for goal BP of <130/80.
Figure 5 ACC/AHA Guideline Recommendation for Duration and Choice of Antiplatelet Agent in Patients with Recent Acute Coronary Syndrome (ACS), Including STEMI Aspirin Therapy is Generally Continued Indefinitely Post-ACS
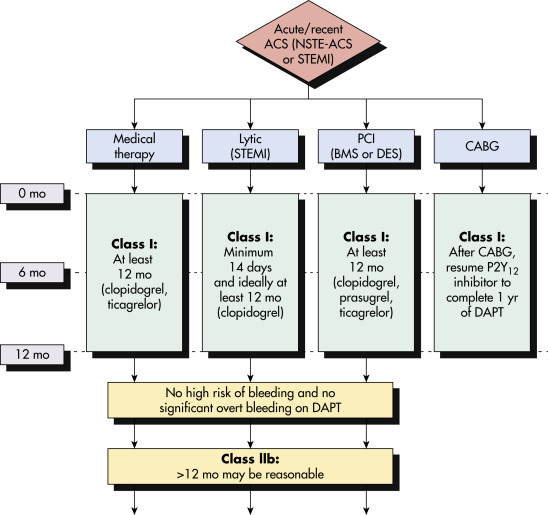
In patients treated with dual-antiplatelet therapy (DAPT) after DES implantation who have a high risk of bleeding (e.g., use of oral anticoagulant therapy, major intracranial surgery) or develop significant overt bleeding, discontinuation of P2Y12 inhibitor therapy after 6 mo for ACS may be reasonable. The optimal duration of prolonged DAPT is not established. BMS, Bare-metal stent; CABG, coronary artery bypass grafting; DES, drug-eluting stent; lytic, fibrinolytic therapy; NSTE-ACS, non-ST-elevation acute coronary syndrome; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction.
Modified from Levine GN et al: 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease, J Am Coll Cardiol 68(10):1082-115, 2016. In Zipes DP: Braunwald’s heart disease, a textbook of cardiovascular medicine, ed 11, Philadelphia, 2019, Elsevier.