AUTHOR: Bharti Rathore, MD
Breast cancer refers to epithelial carcinoma arising from the ducts (ductal) or the lobules (lobular) of the breast that is initially in situ but can progress to become invasive in nature.
Carcinomas make up the majority of breast malignancies and originate in the epithelium of the collecting ducts (ductal) or the terminal lobular ducts (lobular). Sarcomas are rare, constituting less than 1% of primary breast cancers, and arise from stromal or connective tissue. Tumor grade is based on tubule formation, nuclear pleomorphism, and mitotic counts using the Nottingham score to determine low, intermediate, or high grade. Higher grade is associated with a poorer long-term prognosis.
Initially, breast carcinoma may be divided into invasive and in situ lesions (Table 1). Invasive ductal carcinoma accounts for the majority, approximately 80%, of invasive carcinomas. Invasive lobular carcinomas constitute approximately 10% to 15% of cases. Other subtypes include mucinous, tubular, medullary, micropapillary, and papillary. Both in situ and invasive carcinomas are often found in the same quadrant of the breast. Additionally, multifocal carcinomas are not uncommon, and bilateral breast carcinomas occur in 1% to 2% of newly diagnosed cases.
TABLE 1 Simplified Classification of Breast Carcinoma Based on Histology
| Type of Carcinoma | Percentage of All Cases Diagnosed | ||
|---|---|---|---|
| Ductal carcinoma | |||
| In situ | 5 | ||
| Infiltrating | 70 | ||
| Infiltrating with uniform histologic appearance | 10 | ||
| Medullary, colloid, comedo, tubular, papillary | |||
| Lobular carcinoma | |||
| In situ | 3 | ||
| Infiltrating | 9 | ||
| Inflammatory carcinoma | 2 | ||
| Paget disease | 1 | ||
From Gershenson DM et al: Comprehensive gynecology, ed 8, Philadelphia, 2022, Elsevier.
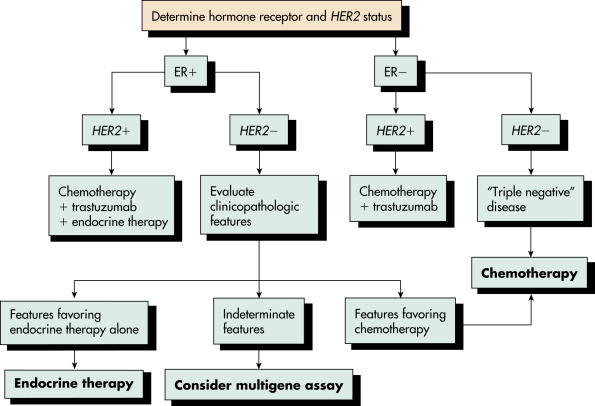
Historically, the treatment of breast cancer was based on tumor histologic characteristics, axillary node status, tumor size, receptor patterns, and grade of differentiation. In addition to simplified histologic classification, a classification based on gene expression or profiling, including the presence of hormone receptors, has evolved. Identification of tumor receptor status is critical because endocrine therapy is used both for adjuvant therapy and in the management of advanced disease. The genomic analysis of tumors has led to the molecular subtyping of breast cancers. In the early 2000s, breast tumors were classified into four different molecular subtypes: luminal, basal, HER2, and normal. Subsequently, the luminal group was further differentiated into luminal-A and luminal-B subgroups. Basal-like tumors include triple-negative tumors, tumors that are estrogen, progesterone, and HER2 negative by immunohistochemistry. A more aggressive subtype of triple-negative tumors, claudin-low tumors, has also been described. These divisions are detailed in Table 2.
TABLE 2 Classification of Breast Carcinoma Based on Gene Profiling and Hormone Receptor
| Expression Type | Grade | Characteristic Behavior | Hormone Receptor Status∗ |
|---|---|---|---|
| Luminal A | Usually low grade | Good prognosis | E and P+ |
| Luminal B | All grades | Mixed prognosis | E and P+, Her2 (Neu)+ |
| Her2 (Neu) | Higher grades | Poor prognosis | E and P-, Her2 (Neu)+ |
| Basal | Usually grade 3 | Poor prognosis | Triple negative |
| Normal breast† | Usually low grade | Good prognosis | Triple negative |
∗E, Estrogen receptor; P, progesterone receptor.
† Normal breast does not express gene profiling of basal elements and myoepithelial gene expression.
From Gershenson DM et al: Comprehensive gynecology, ed 8, Philadelphia, 2022, Elsevier.
| ||||||||||||||||||||||||||||
- In the U.S., there were an estimated 290,560 new patients and an estimated 43,780 deaths in 2022.2
- Hormone-receptor-positive cancers are the most common type and their incidence has increased, particularly among young women.
- Only 1% of breast cancers occur in males.
- Table 3 describes risk factors for breast cancer.
- Factors associated with a decreased risk for breast cancer are summarized in Table 4.
- Known genetic mutations in breast cancer are described in Table 5. Genetically defined group of women with BRCA1 or BRCA2 genes (Table 6) carry lifetime risk as high as 85%.
TABLE 5 Known Genetic Mutations in Breast Cancer and Their Management
| Genetic Mutation | Breast Cancer Risk | Management |
|---|---|---|
| ATM | Increased by 15%-40% | Annual mammography starting at age 40 with consideration for breast tomosynthesis/magnetic resonance imaging (MRI) |
| BARD1 | Limited evidence for increased risk but stronger for triple-negative breast cancer | Annual mammography starting at age 40 with consideration for breast tomosynthesis/MRI |
| BRCA1 BRCA2 | Both carry increased absolute risk greater than 60% | Breast awareness starting at age 18 Clinical breast examination every 6-12 mo starting at age 25 yr Breast screening: Age 25-29 yr: Annual breast MRI screening with contrast or mammogram with consideration of tomosynthesis, only if MRI is unavailable or individualized based on family history if a breast cancer diagnosis before age 30 is present Age 30-75 yr: Annual mammogram with consideration of tomosynthesis and breast MRI screening with contrast Age >75 yr: Management should be individualized Consider risk-reducing mastectomy |
| BRIP1 | Potential increase in risk | Management based on family history |
| CDH1 | Increased absolute risk 41%-60% | Annual mammogram with consideration of tomosynthesis or breast MRI with contrast starting at age 30 |
| CHECK2 | Increased absolute risk 15%-40% | Annual mammography starting at age 40 with consideration for breast tomosynthesis/MRI |
| MSH2 MLH1 MSH6 PMS2 EPCAM | Limited evidence of increased risk, absolute risk is <15% | Management based on family history |
| NBN | Increased risk of breast cancer with variant 657del5 | Management based on family history |
| NF1 | Increased absolute risk 15%-40% | Annual mammography starting at age 30 with consideration for breast tomosynthesis/MRI |
| PALB2 | Increased absolute risk 41%-60% | Annual mammography starting at age 30 with consideration for breast tomosynthesis/MRI Consider risk-reducing mastectomy |
| PTEN | Increased absolute risk 40%-60% | Breast awareness starting at age 18 Clinical breast examination every 6-12 mo starting at age 25 yr (or 5-10 yr before earliest known breast cancer in the family) Breast screening: Age 25-29 yr: Annual breast MRI screening with contrast or mammogram with consideration of tomosynthesis, only if MRI is unavailable or individualized based on family history if a breast cancer diagnosis before age 30 is present Age 30-75 yr: Annual mammogram with consideration of tomosynthesis and breast MRI screening with contrast Age >75 yr: Management should be individualized Consider risk-reducing mastectomy |
| RAD51C | Increased absolute risk 15%-40% | Management based on family history |
| RAD51D | Increased absolute risk 15%-40% | Management based on family history |
| STK11 | Increased absolute risk 40%-60% | Annual mammography alternating with breast MRI every 6 mo starting at age 30 and clinical breast examination every 6 mo |
| TP53 | Increased absolute risk >60% | Breast awareness starting at age 18 Clinical breast examination every 6-12 mo starting at age 20 yr Breast screening: Age 20-29 yr: Annual breast MRI screening with contrast or mammogram with consideration of tomosynthesis, only if MRI is unavailable or individualized based on family history if a breast cancer diagnosis before age 30 is present Age 30-75 yr: Annual mammogram with consideration of tomosynthesis and breast MRI screening with contrast Age >75 yr: Management should be individualized Consider risk-reducing mastectomy |
From Gershenson DM et al: Comprehensive gynecology, ed 8, Philadelphia, 2022, Elsevier.
TABLE 6 Major Inherited Gene Mutation Syndromes Associated With Breast Cancer
| Syndrome | Gene | Incidence | Lifetime Breast Cancer Risk | Associated Cancer Risks |
|---|---|---|---|---|
| BRCA1 | BRCA1 | 1/500-1/1000 | 85% | Ovary and pancreas |
| BRCA2 | BRCA2 | Unclear | 85% | Ovary and pancreas |
| Cowden | PTEN | 1/100,000-1/200,000 | 50% | Thyroid and endometrium |
| Li-Fraumeni | TP53 | 1/20,000 | 90% | Sarcoma, brain, and leukemia |
Other syndromes, including Peutz-Jeghers syndrome, ataxia telangiectasia, CHEK2 gene mutation, and Fanconi syndrome, have much smaller lifetime risks with poorer penetrance.
From Gershenson DM et al: Comprehensive gynecology, ed 8, Philadelphia, 2022, Elsevier.
TABLE 4 Factors Associated With a Decreased Risk for Breast Cancer
| Demographic | Qualification | Relative Risk |
|---|---|---|
| Born and living outside Western countries | ||
| Late menarche | After age 14 | |
| Oophorectomy | Yes vs. no | 0.3 |
| Lactation | >16 mo vs. none | 0.73 |
| Parity | ≥5 vs. 0 | 0.73 |
| Postmenopausal body mass (kg/m2) | <22.9 vs. >30.7 | 0.63 |
| Physical activity | Yes vs. no | 0.70 |
| Vitamin D | Low levels associated with risk | |
| Intake of vitamin D | Associated with decreased risk | |
| Olive oil and omega-3 fatty acids | ||
| Low-fat diet | Results suggestive but not yet conclusive | |
| Aspirin | >1×/wk for ≥16 mo vs. no use | 0.79 |
From Gershenson DM et al: Comprehensive gynecology, ed 8, Philadelphia, 2022, Elsevier.
TABLE 3 Risk Factors for Breast Cancer
| Risk Factor | Qualification | Relative Risk |
|---|---|---|
| Age | ≤49 yr 50-59 yr Age 60-69 yr ≥70 yr | 2.0 2.3 3.5 6.7 |
| Geographic | Common in Western countries | |
| Age at menarche | >14 yr (low risk) vs. <12 yr | 1.5 |
| Age at first full-term pregnancy | <20 yr (low risk) vs. >30 yr | 1.9-3.5 |
| Late menopause | <45 yr (low risk) vs. >55 yr (high risk) | 2.0 |
| Hormone replacement therapy | No use vs. current | 1.2 |
| Contraceptive pill use | None vs. past or current use | 1.07-1.2 |
| Alcohol use | None vs. 2-5 drinks/day | 1.4 |
| Postmenopausal weight gain | Women with a higher body mass index (BMI) | 1.1 per 5 BMI units |
| Bone density | Lowest vs. highest quartile | 2.7-3.5 |
| Nightshift work | Exposed to nightshift work | 1.48 |
| Smoking | History of smoking | 1.10∗ |
| Benign breast disease | None vs. positive biopsy result | 1.7 |
| Breast density (as measured by mammography) | 0% vs. ≥75% | 1.8-6.0 |
| Hyperplasia with atypia | None vs. positive biopsy result | 3.7 |
| Multiple relatives, not first degree, with breast cancer | ||
| One first-degree relative with breast cancer (mother or sister) | None vs. yes | 2.6 |
| Two or more first-degree relatives | Increased risk if the cancers are premenopausal | |
| Deleterious BRCA1/BRCA2 genes | Negative vs. positive | 2.0-7.0 |
| Mantle radiation for treatment of malignancy | Very high risk, which increases with age |
From Gershenson DM et al: Comprehensive gynecology, ed 8, Philadelphia, 2022, Elsevier.
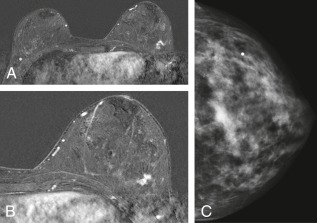
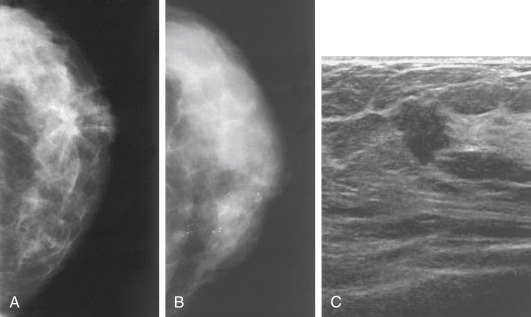
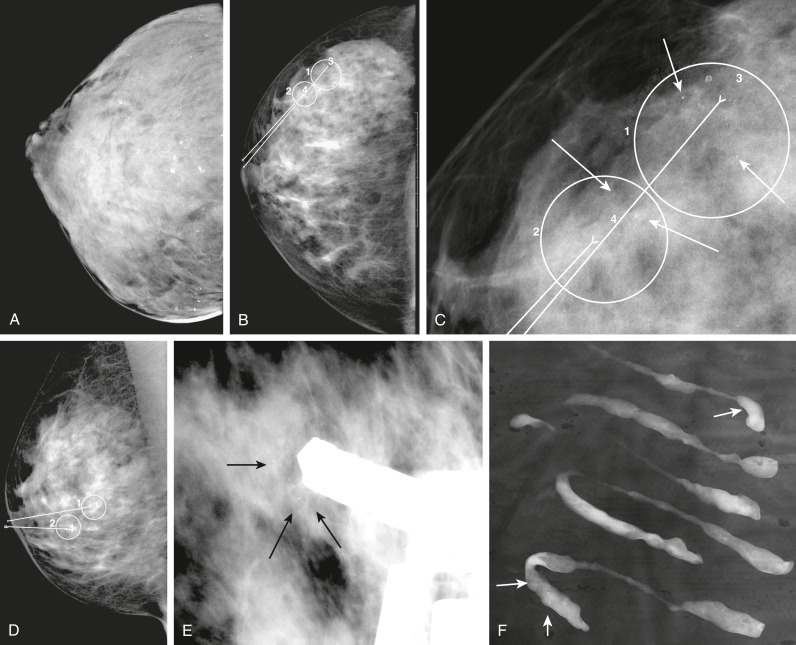
- Increasing number of small breast cancers are found by mammograms, in which case patients are usually completely free of symptoms or physical findings.
- Palpable lump or mass which can be self- or physician-detected.
- Skin and/or nipple retraction and skin edema, erythema, ulcer, satellite nodule.
- Nodal enlargement in axilla and supraclavicular areas.
- Nipple discharge may be serous or bloody.
- Generalized symptoms and signs, including fatigue, weight loss, jaundice, and anorexia, may be present in metastatic cases.
- Endogenous and exogenous estrogen exposure is key to the development of receptor-positive breast cancer
- Breast cancer is no longer perceived as a single disease. Molecular classification based on gene expression profiling has shown breast cancer to be of the following types3:
- Luminal A type: Endocrine responsive with favorable prognosis
- Luminal B type: Endocrine responsive with less favorable prognosis
- Normal type: Resemble normal epithelium and prognosis similar to luminal B type
- HER2 amplified type: HER2 gene amplification occurs in 15% to 20% of cases
- Basal type: Hormone receptor and HER2 negative cancers with poor prognosis
- Approximately 10% of all women with breast cancer have a germline mutation of BRCA1, BRCA2, P53, or other mutations
- Possibly interaction of ovarian estrogen, nonovarian estrogen, estrogens of exogenous origin with breast tissue of varied carcinogenic susceptibility to develop cancer
- Other known or suspected variables: Childbearing, breastfeeding practice, diet, physical activity, body mass, alcohol intake