AUTHOR: Peter J. Mazzaglia, MD
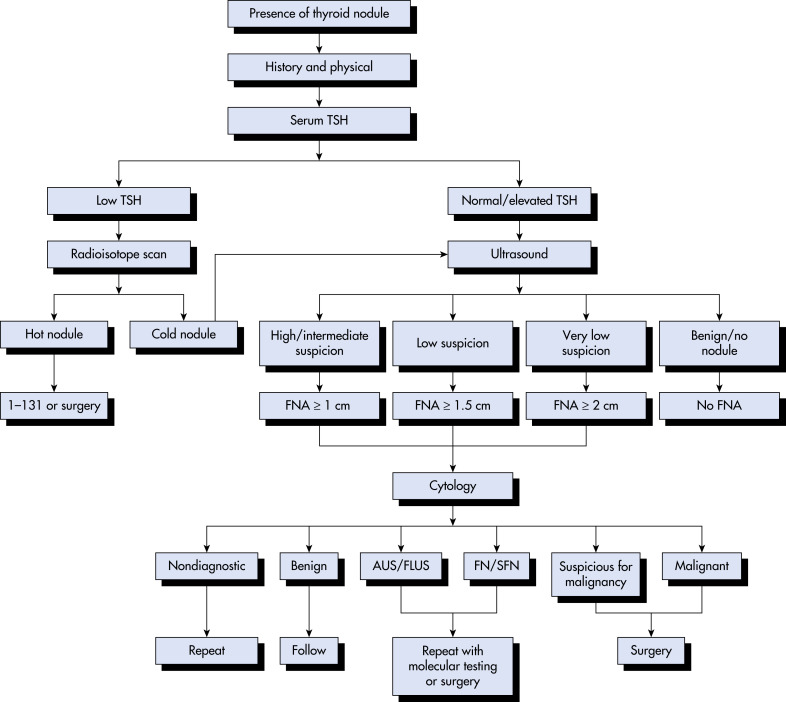
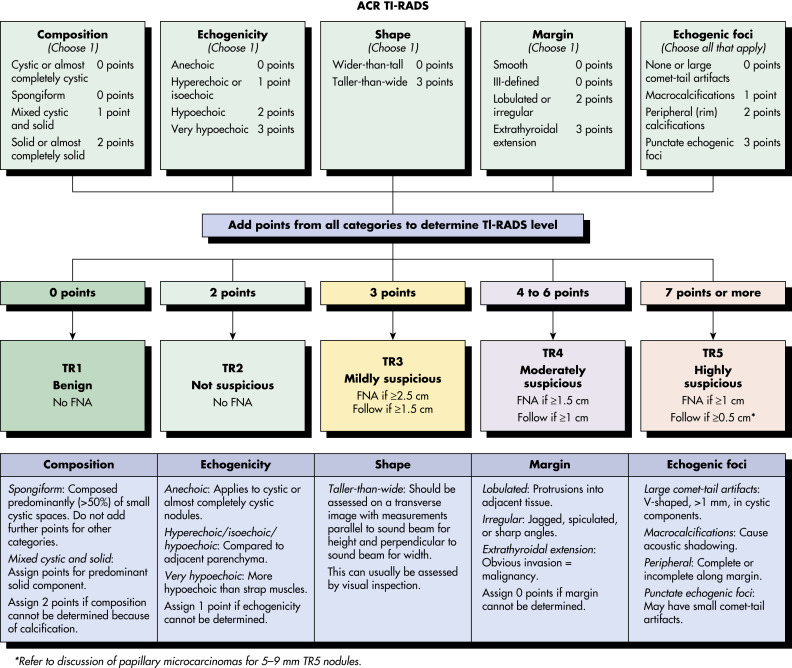
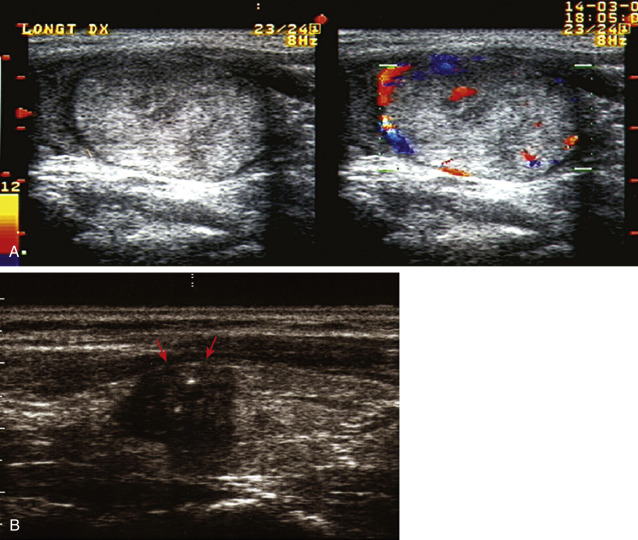
An abnormal growth of thyroid tissue detected on either physical examination or radiographic imaging, and ultimately confirmed by thyroid ultrasound.
| ||||||||||||||||||||||||
- Anatomic: Characteristics of the nodule include size, firmness (ranges from soft to rock hard), mobility, presence of single or multiple nodules, and presence of enlarged cervical lymph nodes. Additional physical findings to look for include exophthalmos, which would suggest Graves disease, tracheal deviation, and hoarseness suggestive of recurrent laryngeal nerve dysfunction usually associated with advanced malignancy.
- Physiologic: Symptoms of thyrotoxicosis that can be seen with a toxic nodule or toxic multinodular goiter include palpitations, anxiety, insomnia, weight loss, and heat intolerance. The signs of thyrotoxicosis include tremor, lid lag, tachycardia, pressured speech, and restlessness.
- Most nodules are benign. They can be solitary or multiple. Positive family history is common. Iodine deficiency is rarely a cause of nodule formation in developed countries, where salt is iodized.
- Malignancy risks include family history of thyroid cancer and prior head and neck irradiation. Indicators of malignancy: Nodule significantly increasing in size, regional lymphadenopathy, fixation to adjacent tissues, very young or very old age at onset, symptoms of local invasion (dysphagia, hoarseness, neck pain), male sex.
- Inherited syndromes: MEN II predisposes to medullary thyroid cancer, and Cowden syndrome predisposes to follicular neoplasms. Other syndromic causes include Carney complex, Gardner syndrome, and familial adenomatous polyposis.