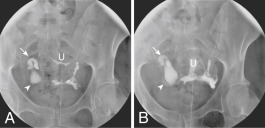
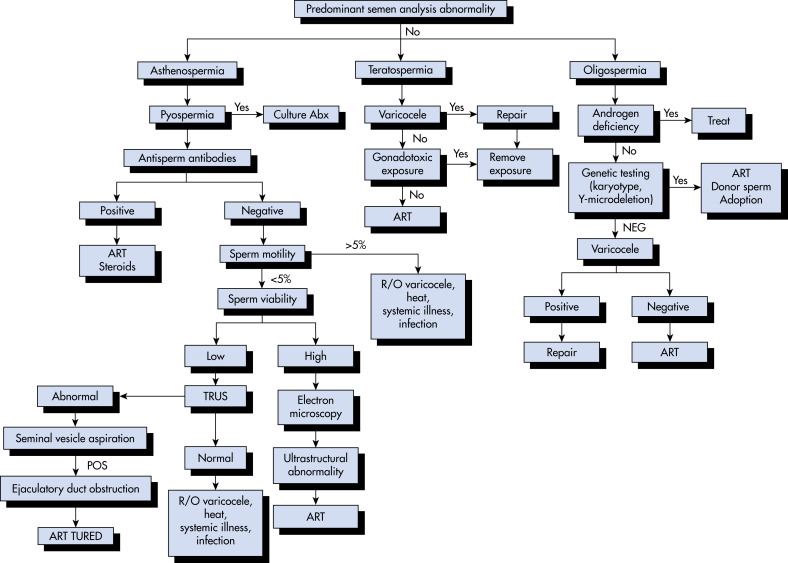
AUTHOR: Emelia Argyropoulos Bachman, MD, FACOG
Infertility in a reproductive-age couple is defined as the inability to conceive after unprotected intercourse for ≥1 yr. When a female is greater than 35 yr of age, an evaluation is recommended after 6 mo without successful pregnancy. Earlier evaluation at any age is warranted with preexisting symptoms or medical conditions.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
One in eight reproductive age couples experience infertility. This prevalence is consistent in all developed countries, and there is evidence that it is historically stable. Infertility affects 8.8% of U.S. women aged 15 to 49 years and approximately 12.7% of reproductive age women seek treatment for infertility each year.1,2
By definition this is a diagnosis of reproductive age couples. Infertility increases with aging in both males and females, but more dramatically in women (Table 1). Male factor is responsible in nearly 40% of couples, and the female factor is responsible in approximately 50% of couples. The remainder of the cases are either combined male and female, or unexplained infertility, meaning a clear cause is not identified.
The incidence of infertility increases with age. Subtle decreases in female fertility start as early as age 30. The rate of infertility increases dramatically after age 37, and unassisted pregnancies become extremely uncommon as women reach the mid-40s. There is also a subtle, but still detectable, decrease in male fertility that may start as early as age 30.
Aging is among the most common risk factors, predominantly among females, although there is evidence that aging affects male fertility as well. Women are increasingly deferring pregnancy due to the lack of a partner or career. Tubal factor infertility can be a result of endometriosis, prior tubal surgery, prior ruptured appendix, or sexually transmitted diseases such as chlamydia and gonorrhea. Ovulatory dysfunction is most commonly caused by polycystic ovarian syndrome (PCOS). Other causes of ovulatory dysfunction include hypothalamic dysfunction, thyroid disorders, hyperprolactinemia, and extremes of weight, particularly obesity. Male factor infertility may be idiopathic or due to trauma, infection, varicocele, obstruction, hypothalamic dysfunction, or exposure to environmental toxins. Smoking is the most common lifestyle choice that impairs fertility.
- Age
- Previous fertility, particularly if no pregnancy has occurred in another relationship despite absence of contraception
- Absence of secondary sexual characteristics
- Abnormal uterine bleeding or absent or irregular menstruation
- Clinical signs of androgen excess: Hirsutism, acne, alopecia
- Abnormal pelvic exam: Enlarged uterus, adnexal masses, pelvic/abdominal tenderness
- History of urologic surgery in male or trauma to testes
- Female factor:
- Advanced age
- Tubal factor: Pelvic inflammatory disease, endometriosis, prior pelvic surgery, history of ruptured appendicitis, prior elective sterilization
- Anatomic: Uterine fibroids, polyps, intrauterine adhesions, congenital uterine anomalies
- Oligo-/anovulation: Most frequently due to polycystic ovarian syndrome (PCOS), but also due to thyroid abnormalities, hyperprolactinemia, nonclassic congenital adrenal hyperplasia, or hypothalamic dysfunction
- Male factor (Table 2):
- Idiopathic: Both male and female
TABLE 2 Causes of Male Infertility
| Cause | Examples | ||
|---|---|---|---|
| Hypogonadism | |||
| Isolated impairment of sperm production or function | |||
| Androgen deficiency and impaired sperm production | |||
| Androgen resistance | |||
| Disorders of Sperm Transport | |||
| Genital tract obstruction | Congenital bilateral absence of the vas deferens, cystic fibrosis, other congenital defects, vasectomy, postinfectious fibrosis, Young syndrome | ||
| Accessory gland dysfunction | Androgen deficiency or resistance, infection or inflammation, antisperm antibodies (immunologic) | ||
| SNS dysfunction | Autonomic neuropathy, sympatholytic drugs, sympathectomy, retroperitoneal or abdominopelvic surgery, spinal cord injury or disease, vasovasostomy | ||
| Ejaculatory Dysfunction | |||
| Premature or retarded ejaculation | |||
| Retrograde ejaculation | Prostatectomy, bladder neck surgery, autonomic neuropathy, SNS dysfunction | ||
| Reduced ejaculation | Androgen deficiency or resistance, SNS dysfunction, ureteral abnormalities | ||
| Coital Disorders | |||
| Erectile dysfunction | |||
| Defects in coital technique | Infrequent intercourse (<once weekly), poor timing in relation to ovulation, premature withdrawal of penis | ||
SNS, Sympathetic nervous system.
From Melmed S et al: Williams textbook of endocrinology, ed 14, Philadelphia, 2019, Elsevier.
TABLE E3 Fertility Preservation Options Among Females
| Option | Embryo Freezing | Egg Freezing | Ovarian Tissue Freezing | Radiation Shielding of Gonads | Ovarian Transposition | Radical Trachelectomy | Ovarian Suppression |
|---|---|---|---|---|---|---|---|
| Medical status | Standard | Standard | Standard | Standard | Standard | Standard | Experimental |
| Definition | Harvesting eggs, in vitro fertilization, and freezing of embryos for later implantation | Harvesting and freezing of unfertilized eggs | Freezing of ovarian tissue and reimplantation after cancer treatment | Use of shielding to reduce scatter radiation to the reproductive organs | Surgical repositioning of ovaries away from the radiation field | Surgical removal of the cervix with preservation of the uterus | Gonadotropin-releasing hormone analogs or antagonists used to suppress ovaries |
| Pubertal status | After puberty | After puberty | Before or after puberty | Before or after puberty | Before or after puberty | After puberty | After puberty |
| Time requirement | 10-14 days; outpatient surgical procedure | 10-14 days; outpatient surgical procedure | Outpatient surgical procedure | In conjunction with radiation treatments | Outpatient procedure | Inpatient surgical procedure | In conjunction with chemotherapy |
| Success rates | Approximately 40% per embryo transfer; varies by age and center | Approximately 4%-6% live birth per oocyte; over 5000 live births worldwide | Varies by age. Over 200 live births worldwide | Only possible with selected radiation fields and anatomy | Approximately 50% because of altered blood flow and scattered radiation | No evidence of higher cancer recurrence rates in appropriate candidates | Unknown; conflicting results reported; larger randomized trials in progress |
| Cost | Approx. $10,000-$12,000/cycle; storage fees additional | Approx. $5500/cycle; storage fees additional | Approx. $10,000 for procedure; storage fees and reimplantation costs additional | Generally included in cost of radiation | May be covered by insurance if performed at the time of another procedure | Generally included in the cost of cancer treatment | Approx. $500/mo, but may be covered in the cost of chemotherapy |
| Timing | Ideally before treatment, but may perform after depending on ovarian reserve | Ideally before treatment, but may perform after depending on ovarian reserve | Ideally before treatment, but may perform after depending on ovarian reserve | During treatment | Before treatment | During treatment | Start before and continue through treatment |
| Special considerations | Need partner or donor sperm | Beneficial for single women or those with ethical concerns regarding embryo creation | Not suitable if high risk of ovarian metastases; only preservation option for prepubescent girls | Expertise required; does not protect against effects of chemotherapy | Expertise required | Limited to early-stage cervical cancer; offered at a limited number of centers | Does not protect from radiation effects |
Modified from https://www.livestrong.org/fertility. In Niederhuber JE: Abeloff’s clinical oncology, ed 6, Philadelphia, 2020, Elsevier.
Once the patient presents for evaluation, testing should be completed as quickly as possible, ideally within one menstrual cycle. The couple should follow up with the evaluating provider once all testing is completed, and treatment should be initiated as abnormalities are found.