AUTHORS: Natasha Choudhury, MD and Corey Elam Goldsmith, MD, FAAN
Multiple sclerosis (MS) is a chronic, predominantly autoimmune demyelinating disease of the central nervous system (CNS), characterized by subacute neurologic deficits correlating with CNS lesions (typical for MS in location, shape, and orientation) separated in time (typically at least 1 mo) and space, and excluding other possible disease.1-3
An MS relapse is defined as an acute to subacute (peaking over hours to days) onset of neurologic dysfunction (typically focal) lasting at least 24 h, and caused by inflammatory CNS demyelination. Relapses can be symptomatic or asymptomatic, the latter of which are represented by new enhancing MRI lesions without correlating symptoms.
- Relapsing-remitting MS (RRMS) (85%): Relapses followed by complete or near-complete recovery over weeks to months (rarely beyond 6 mo), 50% to 85% of which later evolve to secondary progressive MS.2-3
- Primary progressive MS (PPMS) (10% to 15%): Progressive worsening of neurologic disability from the onset, with rare distinct relapses.2-3
- Secondary progressive MS (SPMS): Progressive worsening of neurologic disability over at least 1 yr with few or no distinct relapses, from a prior course consistent with RRMS.
- Clinically isolated syndrome (CIS): An initial, isolated clinical event lasting at least 24 h and typical for MS relapse, but not yet meeting criteria for dissemination in time and space. Correlating demyelinating lesions on MRI are associated with 60% to 80% risk of developing a second relapse (and therefore, MS) within several years, vs. 20% without the presence of typical MS lesions on MRI.2-3 The definition for RRMS is met when another distinct relapse occurs or when MRI demonstrates new lesions typical for MS.2,4
- Radiologically isolated syndrome (RIS): The presence of CNS lesions on MRI that meet the diagnostic imaging criteria for MS, without correlating symptoms or symptoms typical for MS. Approximately 50% develop MS within 10 years.1,2
- Solitary sclerosis: Characterized by isolated or minimal CNS demyelinating lesion(s) with associated progressive neurologic morbidity similar to that in progressive MS, without any clinical or radiologic evidence of new MS-type lesions.2
- Marburg variant: Characterized by acute onset and a fulminant, often malignant course (severe disability or death can occur within a year of initial symptoms). MRI reveals tumefactive (tumor-like) demyelinating lesion(s) with extensive edema. Pathology shows severe inflammation with extensive necrosis. May also involve the peripheral nerves.5
- Baló’s concentric sclerosis: Has a monophasic and rapidly progressive course. Neuroimaging and pathology show alternating rings of high- and low-signal intensity (on MRI) representing demyelination and intact myelination, resembling an onion bulb.5 More common in those of Chinese and Filipino descents.
- Schilder’s disease (myelinoclastic diffuse sclerosis): Onset is typically in childhood with 1 to 2 large, confluent lesions. Usually progresses to involve widespread, bilateral regions of the CNS, and has variable course and prognosis.6-7 Etiology is unclear but may have a possible association with preceding infectious illness.
More common in people raised in northern latitudes and in certain genetic clusters.1 Global prevalence of MS is estimated to be around 36 per 100,000 people (∼3 million people).8 There are currently nearly 1 million people living with MS in the United States.9
Most common permanently disabling disorder of the central nervous system in young adults.1 Two thirds of patients have incidence between 20 and 40 yr; mean age of onset is 30 yr. Ranges from infancy to 70 yr.10
Although more common in White people of Northern European descent, MS has been demonstrated to occur across most races and ethnic backgrounds. Recent studies suggest up to 47% higher risk of MS in Black vs. White women in the United States. Hispanic, Asian, and Native American populations have demonstrated lower incidence of MS thus far, compared to White and Black populations. Features of MS that have been more commonly associated with Black patients (compared to White patients) include transverse myelitis, vision loss from optic neuritis, earlier progression to disability, and higher lesion burden. Hispanic patients may also more frequently have optic neuritis and transverse myelitis, as well as younger age of disease onset and more severe disease course. Asian patients may have higher rates of optic nerve and spinal cord involvement.11
Frequency of MS in dizygotic twins and siblings is 3% to 5%, and 30% to 50% in monozygotic twins. ∼200 genes have been identified that contribute to the risk of developing MS.1 Most common associations include human leukocyte antigen classes I and II (DRB1∗1501, DQA1∗0102, DQB1∗0602), (DRB1∗0405-DQA1∗0301-DQB1∗0302 in the Mediterranean population). A notable epigenetic interaction between vitamin D and the main MS-linked HLA-DRB1∗1501 allele has been elucidated. Conversely, HLA-A∗02 has been associated with a reduced odds of developing MS.12
Findings depend on the location of the CNS lesion(s) and may include the following:
- Common: Nonspecific complaints such as fatigue (most common, with 80% lifetime prevalence), blurred vision, diplopia, vertigo, falls, hemiparesis, paraparesis, monoparesis, numbness, paresthesias, ataxia, cognitive impairment, depression, anxiety, pseudobulbar affect (involuntary crying or laughing out of context), sexual dysfunction, and bowel/bladder dysfunction
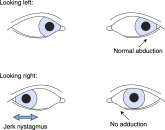
- Visual abnormalities: Horizontal nystagmus, visual field deficits, Marcus Gunn pupil (i.e., relative afferent papillary defect-normal consensual light reflex; however, when swinging a flashlight from the unaffected eye to the affected eye, direct light causes paradoxical pupillary dilation in the affected eye), sixth nerve palsy, internuclear ophthalmoplegia (paresis of the adducting eye on conjugate lateral gaze with simultaneous horizontal nystagmus of the abducting eye) (Fig. 1)
- Corticospinal tract(s) involvement: Transverse myelitis, upper motor neuron signs such as spasticity (particularly leg spasms at night or after prolonged immobility), hyperreflexia, clonus, extensor plantar responses, tonic spasms, upper motor neuron pattern of weakness
- Sensory involvement: May include partial or full dermatomal loss of pain and temperature, loss of vibration (common) and position sense, temperature dysregulation, thoracic band of sensory loss, paresthesias, trigeminal neuralgia
- Ataxia: Intention tremor, dysmetria, dysdiadochokinesis, titubation, inability to tandem gait
- Bladder dysfunction: Detrusor hyperreflexia (urge incontinence), urinary frequency, flaccidity (neurogenic bladder), and dyssynergia (bladder contracts against a closed sphincter)
- Lhermitte sign: Flexion of the neck elicits an electrical sensation extending down the spine and occasionally into the extremities, due to involvement of the posterior cervical spinal cord2,13
- Uhthoff phenomenon: Transient recurrence or worsening of preexisting neurologic deficits with small elevations in core body temperature (e.g., during exercise or warm bathing)2
Figure 1 Internuclear ophthalmoplegia.
When the patient in the figure looks to the left (top row), both eyes move normally, but when the patient looks to the right (bottom row), the left eye fails to adduct (“weak” medial rectus) and the contralateral eye develops a jerk nystagmus. The finding is named for the side with weak adduction (i.e., in this example, a left internuclear ophthalmoplegia), and the lesion is in the ipsilateral medial longitudinal fasciculus (i.e., left medial longitudinal fasciculus in this example). See the text.
From McGee S: Evidence-based physical diagnosis, ed 4, Philadelphia, 2018, Elsevier.
Likely multifactorial, with evidence for autoimmunity (autoreactive T and B lymphocytes), environmental factors (low sunlight exposure, vitamin D deficiency, smoking, obesity, shift work), and genetics (Mendelian and epigenetic). Environmental risk factors during childhood include exposure to certain viruses (e.g., Epstein-Barr virus and human herpes virus 6), low ultraviolet light exposure, and month of birth (higher in spring).1,14