Author: Luis Osorio, DO and Hesham Shaban, MD
- Tumor lysis syndrome (TLS) is an oncologic emergency referring to the acute release of potentially injurious intracellular contents into the systemic circulation from tumor cell breakdown.
- TLS is a constellation of metabolic disturbances that typically occur in the setting of rapid tumor cell lysis induced by cancer-targeted treatment (chemotherapy and/or other interventions such as embolization or radiation). However, spontaneous tumor cell turnover has produced TLS (i.e., no chemotherapy administration).
- TLS is characterized by hyperuricemia, hyperphosphatemia, hypocalcemia, hyperkalemia, and acute kidney injury (AKI), often with progressive oliguria.2,3
- In the current classification system by Cairo and Bishop, TLS may be defined by laboratory or clinical parameters (Table E1). Grading of clinical TLS is summarized in Table E2.5
- Laboratory TLS: Clinically silent; defined by at least two of the following biochemical variables within 3 days before or 7 days after initiation of chemotherapy, despite adequate volume status and uric acid-lowering agents.
- Hyperuricemia:
- Hyperkalemia
- Hyperphosphatemia: Phosphate may precipitate with calcium to form calcium phosphate stones in renal tubules
- Hypocalcemia: Occurs secondary to precipitation with phosphorus
- Clinical TLS occurs when laboratory TLS is complicated by at least one of the following clinical complications that is not attributed to the chemotherapeutic regimen: Severe renal impairment, cardiac arrhythmias, central nervous system toxicity, and/or death.
- Laboratory TLS: Clinically silent; defined by at least two of the following biochemical variables within 3 days before or 7 days after initiation of chemotherapy, despite adequate volume status and uric acid-lowering agents.
TABLE E1 Classification of Tumor Lysis Syndrome
From Niederhuber JE: Abeloff’s clinical oncology, ed 6, Philadelphia, 2020, Elsevier.
TABLE E2 Grading of Clinical Tumor Lysis Syndrome
| Stage I | Stage II | Stage III | Stage IV | Stage V |
|---|---|---|---|---|
| Renal failure | Serum creatinine = 1.5 UNL or creatinine clearance 30-45 ml/min | Serum creatinine = 1.5-3 UNL or creatinine clearance 20-30 ml/min | Serum creatinine = 3-6 UNL or creatinine clearance 10-20 ml/min | Serum creatinine >6 UNL or creatinine clearance <10 ml/min |
| Cardiac arrhythmia | Intervention not indicated | Nonurgent intervention indicated | Symptomatic and incompletely controlled or controlled with device (e.g., defibrillator) | Life-threatening (e.g., arrhythmia associated with heart failure, hypotension, syncope, shock) |
| Seizures | None | One brief, generalized seizure; seizure(s) well controlled by anticonvulsant, or infrequent focal motor seizures | Seizure in which consciousness is altered; poorly controlled seizure disorder, with generalized breakthrough seizures despite medical intervention | Seizure of any kind that is prolonged, repetitive, or difficult to control (e.g., status epilepticus, intractable epilepsy) |
From Ronco C et al: Critical care nephrology, ed 3, Philadelphia, 2019, Elsevier.
The frequency of TLS is unknown, but it is the most common disease-related emergency encountered by physicians who treat children or adults with hematologic cancers. Incidence depends on cancer mass, patient characteristics (e.g., preexisting chronic kidney disease, volume depletion, hypotension), and supportive care. Tumor bulk, proliferation rate, and treatment sensitivity are associated with greater frequency of TLS.3,4
- Large tumor burden:
- Administration of certain agents:
- Extensive bone marrow involvement
- Elevated pretreatment uric acid, potassium, or phosphorus levels
- Tumor that is highly sensitive to treatment
- Volume depletion
- Chronic kidney disease
- Decreased urine output
- Acidic urine
- Tumor involvement of the renal vasculature
- Advanced age
- Posttranscatheter arterial chemoembolization (TACE), radiofrequency thermal ablation
- Chimeric antigen receptor T-cell therapy (CAR-T)
Patients may present with a number of symptoms before starting chemotherapy or commonly within 3 days after initiating cytotoxic treatment. Common symptoms include any of the following:
- Nausea
- Vomiting
- Edema
- Shortness of breath (from fluid overload or congestive heart failure)
- Lethargy or weakness
- Seizure
- Syncope
- Muscle cramp
- Tetany
Most commonly occurs in patients with acute leukemias; bulky, solid tumors; or high-grade lymphomas. The pathophysiology of TLS is illustrated in Fig. E1.
- Can occur spontaneously or after antitumor intervention (chemotherapy, radiation, etc.)
- Associated with administration of certain chemotherapeutic agents (whether intravenous, intrathecal, etc.):
- Spontaneous TLS
- Rare
- Lysis of tumor cells without chemotherapy or in setting of minimal chemotherapy (e.g., steroid monotherapy lymphoma)
- Related to rapidity of cell turnover
- Associations with pregnancy, fever, and rarely, general anesthesia in predisposed individuals
- Hyperphosphatemia may not recur because of the reutilization of released phosphorus for resynthesis of newer tumor cells
Figure E1 Pathophysiology of tumor lysis syndrome (TLS).
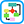
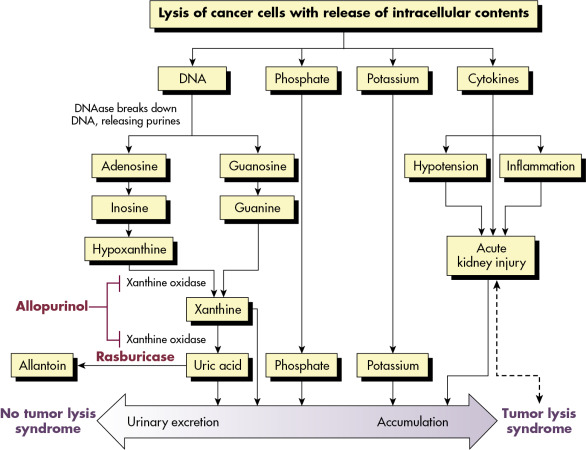
Lysis of cancer cells releases DNA, phosphate, potassium, and cytokines. DNA released from the lysed cells is metabolized into adenosine and guanosine, both of which are converted into xanthine. Xanthine is then oxidized by xanthine oxidase, leading to the production of uric acid, which is excreted by the kidneys. When the accumulation of phosphate, potassium, xanthine, or uric acid is more rapid than excretion, tumor lysis syndrome develops. Cytokines cause hypotension, inflammation, and acute kidney injury (AKI), which increases the risk for TLS. The bidirectional dashed line between AKI and tumor lysis syndrome indicates that AKI increases the risk of tumor lysis syndrome by reducing the ability of the kidneys to excrete uric acid, xanthine, phosphate, and potassium. By the same token, development of tumor lysis syndrome can cause AKI by renal precipitation of uric acid, xanthine, and calcium phosphate crystals and by crystal-independent mechanisms. Allopurinol inhibits xanthine oxidase and prevents the conversion of hypoxanthine and xanthine into uric acid but does not remove existing uric acid. In contrast, rasburicase removes uric acid by enzymatically degrading it into allantoin, a highly soluble product that has no known adverse effects on health.
(From Niederhuber JE: Abeloff’s clinical oncology, ed 6, Philadelphia, 2020, Elsevier.)