| ESSENTIALS OF DIAGNOSIS | ||
|
General Considerations
Primary hyperparathyroidism is the most common cause of hypercalcemia, with an estimated prevalence of 0.89% of the US population, but it is underdiagnosed and undertreated. It occurs at all ages but most commonly in the seventh decade and in women (74%). Before age 45, the prevalence is similar in men and women. It is more prevalent in Black adults than in other races.
Parathyroid glands vary in number and location, and ectopic parathyroid glands have been found within the thyroid gland, high in the neck or carotid sheath, the retroesophageal space, and the thymus or mediastinum. Hyperparathyroidism is usually caused by a single parathyroid adenoma (80%), and less commonly by hyperplasia or adenomas of two or more parathyroid glands (20%), or carcinoma (less than 1%). When hyperparathyroidism presents before age 30 years, there is a higher incidence of multiglandular disease (36%) and parathyroid carcinoma (5%). The size of the parathyroid adenoma correlates with the serum PTH level.
Hyperparathyroidism is familial in about 5-10% of cases; hyperparathyroidism presenting before age 45 has a higher chance of being familial. Parathyroid hyperplasia commonly arises in MEN types 1, 2 (2A), and 4. (See Table 28-12. Multiple Endocrine Neoplasia (MEN) Syndromes: Incidence of Tumor Types.) In MEN 1, multiglandular hyperparathyroidism is usually the initial manifestation and ultimately occurs in 90% of affected individuals. Hyperparathyroidism in MEN 2 (2A) is less frequent than in MEN 1 and is usually milder. Hyperparathyroidism-jaw tumor syndrome is a familial autosomal dominant condition with parathyroid adenomas or carcinomas that are associated with tumors of the jaw and renal lesions. Familial isolated hyperparathyroidism refers to familial hyperparathyroidism without the characteristic extraparathyroid features of other more complex hyperparathyroid syndromes. Affected kindreds may harbor an incomplete phenotypic expression of MEN 1 or hyperparathyroidism-jaw tumor syndrome, or germline mutations in other genes, such as CASR (encoding the calcium sensing receptor), CDC73, or GCM2.
Table 28-12. Multiple endocrine neoplasia (MEN) syndromes: incidence of tumor types.Tumor Type | MEN 1 | MEN 2 (MEN 2A) | MEN 3 (MEN 2B) | MEN 4 |
|---|---|---|---|---|
Parathyroid | 95% | 20-50% | Rare | Common |
Pancreatic | 54% |
|
| Common |
Pituitary | 42% |
|
| Common |
Medullary thyroid carcinoma |
| >90% | 80% |
|
Pheochromocytoma | Rare | 20-35% | 60% |
|
Mucosal and GI ganglioneuromas |
| Rare | >90% |
|
Subcutaneous lipoma | 30% |
|
|
|
Adrenocortical adenoma | 30% |
|
| Common |
Thoracic carcinoid | 15% |
|
|
|
Thyroid adenoma | 55% |
|
| Common |
Facial angiofibromas and collagenomas | 85% |
|
|
|
Breast cancer | 27% |
|
|
|
Hyperparathyroidism results in the excessive excretion of calcium and phosphate by the kidneys. PTH stimulates renal tubular reabsorption of calcium; however, hypercalcemia resulting in increase in calcium in the glomerular filtrate overwhelms tubular reabsorption capacity, resulting in hypercalciuria. At least 5% of renal calculi are associated with this disease. Diffuse parenchymal calcification (nephrocalcinosis) is seen less commonly.
Parathyroid carcinoma is a rare cause of hyperparathyroidism, accounting for less than 1% of hyperparathyroidism. However, it occurs in about 15% of patients with hyperparathyroidism jaw-tumor syndrome and is also more common in patients with familial hyperparathyroidism or MEN. Surgical histopathology typically reveals prominent fibrous bands (90%), capsular invasion (60%), vascular invasion (15%), high mitotic index (80%), and reduced staining for parafibromin (67%). Local recurrence is the rule if surgical margins are positive. Distant metastases arise most commonly in the lungs but also in bones, liver, brain, and mediastinum. Although parathyroid carcinoma is typically indolent, an increasing tumor burden is associated with critically severe hypercalcemia and death.
Secondary and tertiary hyperparathyroidism usually occurs with CKD, in which hyperphosphatemia and decreased renal production of 1,25-dihydroxycholecalciferol (1,25[OH]2D3) frequently result in a decrease in ionized calcium. The parathyroid glands are stimulated by the hypocalcemia (secondary hyperparathyroidism) and over time may become enlarged and autonomous (tertiary hyperparathyroidism). Renal osteodystrophy is the bone disease of this disorder (see Disorders of Mineral Metabolism, Part 24). Following kidney transplant, persistent hyperparathyroidism may cause hypercalcemia and hypophosphatemia. Hypercalcemia often occurs after kidney transplant. Secondary hyperparathyroidism also develops in patients with a deficiency in vitamin D. Serum calcium levels are typically in the normal range but may become borderline elevated over time with tertiary hyperparathyroidism. (See Osteomalacia.)
Clinical Findings
A. Symptoms and Signs
Hypercalcemia is often discovered incidentally by routine chemistry panels. Many patients are asymptomatic or have only mild symptoms. Parathyroid adenomas are usually small, located deeply in the neck, and almost never palpable; when a mass is palpated, it usually turns out to be an incidental thyroid nodule.
Symptomatic patients are said to have problems with "bones, stones, abdominal groans, psychic moans, with fatigue overtones."
1. Skeletal Manifestations
Low bone density is typically most prominent at the distal one-third of the radius, a site of mostly cortical bone. Lumbar (trabecular) spine bone density is often spared and is higher compared to the distal radius. Hip bones are a mixture of trabecular and cortical bone, and the risk for hip fractures is increased by 50%. Postmenopausal women are prone to asymptomatic vertebral fractures, but severe vertebral demineralization is uncommon in mild hyperparathyroidism. More commonly, patients experience arthralgias and bone pain, particularly involving the legs. Severe chronic hyperparathyroidism can cause osteitis fibrosa cystica, which is the replacement of calcified bone matrix with fibrous tissue forming cystic brown tumors of bone that can be palpable in the jaw. The skeleton becomes weaker with bowing of the long bones and pathologic fractures. Osteitis fibrosa cystica is a presenting manifestation in under 2% of hyperparathyroidism patients in the United States, Canada, and Europe while worldwide, it remains more common (eFigure 28-15) (eFigure 28-16).
eFigure 28-15. Osteitis Fibrosa Cystica with Resorption of the Distal Phalanges
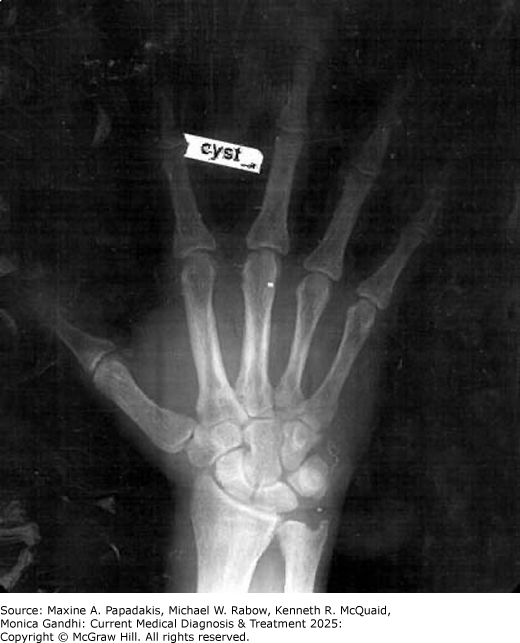
Osteitis fibrosa cystica with resorption of the distal phalanges. (Reproduced with permission from Carl Grunfeld, MD.)
eFigure 28-16. Radiographs of Index Finger of a Patient before (Left) and after (Right) Plasma Phosphate Was Reduced to Normal with Aluminum Hydroxide Gel and 6 Months of Dialysis Against a Bath of 8 mg/dL Calcium

Radiographs of index finger of a patient before (left) and after (right) plasma phosphate was reduced to normal with aluminum hydroxide gel and 6 months of dialysis against a bath of 8 mg/dL calcium. (Reproduced, with permission, from Vosik WM et al. Successful medical management of osteitis fibrosa due to tertiary hyperparathyroidism. Mayo Clin Proc. 1972:47:110.)
2. Hypercalcemic Manifestations
Mild hypercalcemia may be asymptomatic. However, symptom severity is not entirely predicted by the level of serum calcium or PTH; even mild hypercalcemia can cause significant symptoms, particularly depression, constipation, and bone and joint pain. Neuromuscular manifestations include paresthesias, muscle cramps and weakness, and diminished deep tendon reflexes. Neuropsychiatric manifestations include malaise, headache, fatigue, insomnia, irritability, and depression. Patients may have cognitive impairment that can vary from intellectual weariness to severe disorientation, psychosis, or stupor. Cardiovascular manifestations include hypertension, palpitations, prolonged P-R interval, shortened Q-T interval, bradyarrhythmias, heart block, asystole, and sensitivity to digitalis. Overall cardiovascular mortality is increased in patients with chronic moderate to severe hypercalcemia. Renal manifestations include polyuria and polydipsia from hypercalcemia-induced nephrogenic diabetes insipidus. Among patients with newly discovered hyperparathyroidism, calcium-containing renal calculi have occurred or are detectable in about 18%. Patients with asymptomatic hyperparathyroidism have a 5% incidence of asymptomatic calcium nephrolithiasis. GI symptoms include anorexia, nausea, heartburn, vomiting, abdominal pain, weight loss, constipation, and obstipation. Pancreatitis occurs in 3%. Dermatologic symptoms may include pruritus. Calcium may precipitate in the corneas ("band keratopathy"), in extravascular tissues (calcinosis), and in small arteries, causing small vessel thrombosis and skin necrosis (calciphylaxis).
3. Normocalcemic Primary Hyperparathyroidism
Patients with normocalcemic primary hyperparathyroidism generally have few symptoms. They may have a more atherogenic lipid panel and higher blood pressures (systolic blood pressure 10 mm Hg higher and diastolic blood pressure 7 mm Hg higher) than controls. Affected patients can have subtle symptoms, such as mild fatigue, that may not be appreciated.
4. Hyperparathyroidism During Pregnancy
Pregnant women having mild hyperparathyroidism with a serum calcium below 11.0 mg/dL (less than 2.75 mmol/L) generally tolerate pregnancy well with normal outcomes. However, pregnant women with more severe hypercalcemia may experience complications such as nephrolithiasis, hyperemesis, pancreatitis, muscle weakness, and cognitive changes. About 30% of affected women experience preeclampsia and two-thirds of eclamptic women have preterm delivery. Hypercalcemic crisis may occur, especially postpartum. About 80% of fetuses experience complications of maternal hyperparathyroidism, including fetal demise, preterm delivery, and low birth weight. Newborns have hypoparathyroidism that can be permanent.
5. Parathyroid Carcinoma
Hyperparathyroidism with a large palpable neck mass, or vocal fold paralysis from recurrent laryngeal nerve palsy, raises concern for parathyroid carcinoma. Some cases present with smaller tumors, less severe hypercalcemia, and benign-appearing histologic features. FNA biopsy is not recommended because it may seed the biopsy tract with tumor and cytologic distinction between benign and malignant tumors is problematic. Parathyroid carcinoma is more frequent in patients with hyperparathyroidism-jaw tumor syndrome as well as patients with MEN 1 and MEN 2A. Therefore, patients should have genetic testing.
B. Laboratory Findings
The hallmark of primary hyperparathyroidism is hypercalcemia, with the serum adjusted total calcium greater than 10.5 mg/dL (2.6 mmol/L) (eFigure 28-17). The adjusted total calcium = measured serum calcium in mg/dL + [0.8 × (4.0 - patient's serum albumin in g/dL)]. Serum ionized calcium levels are elevated (above 1.36 mmol/L).
eFigure 28-17. Parathyroid Hormone and Calcium Nomogram
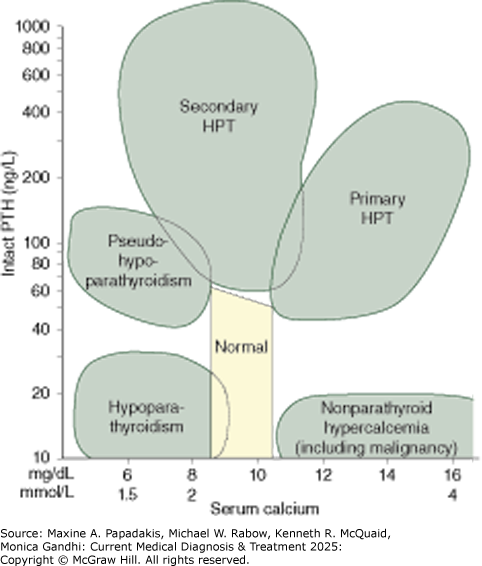
Parathyroid hormone and calcium nomogram. Relationship between serum intact parathyroid hormone (PTH) and serum calcium levels in patients with hypoparathyroidism, pseudohypoparathyroidism, nonparathyroid hypercalcemia, primary hyperparathyroidism (HPT), and secondary hyperparathyroidism. (Reproduced with permission from GJ Strewler, MD.) Note: A multivariate model that adds clinical and demographic information may perform better than the nomogram alone. (See O'Neill SS et al. Multivariate analysis of clinical, demographic, and laboratory data for classification of disorders of calcium homeostasis. Am J Clin Pathol. 2011 Jan;135(1):100-7. [PMID: 21173131])
To confirm the diagnosis of hyperparathyroidism, assess urinary calcium excretion. In primary hyperparathyroidism, the urine calcium excretion is normal (100-300 mg/day [25-75 mmol/day]) or high. Low urine calcium excretions (below 100 mg/day [25 mmol/day]) in the absence of thiazide diuretics occur in only 4% of cases of primary hyperthyroidism and raise the differential diagnosis of familial hypocalciuric hypercalcemia.
In primary hyperparathyroidism, serum phosphate may be less than 2.5 mg/dL (0.8 mmol/L) due to an excessive loss of phosphate in the urine (25% of cases). A serum calcium:phosphate (Ca/P) ratio above 2.5 (mg/dL) or above 2.17 (mmol/L) helps confirm the diagnosis of primary hyperparathyroidism. The alkaline phosphatase is elevated only if bone disease is present. The plasma chloride and uric acid levels may be elevated. Serum 25-OH vitamin D levels should be measured, since vitamin D deficiency is common in patients with hyperparathyroidism. Serum 25-OH vitamin D levels below 20 mcg/L (50 nmol/L) can aggravate hyperparathyroidism and its bone manifestations.
Elevated serum levels of intact PTH confirm the diagnosis of hyperparathyroidism. Parathyroid carcinoma must always be suspected in patients with a serum calcium of 14.0 mcg/dL (3.5 mmol/L) or more and a serum PTH 5 or more times the upper limit of normal.
Patients with low bone density, normal serum calcium, and an elevated serum PTH must be evaluated for causes of secondary hyperparathyroidism (eg, vitamin D or calcium deficiency, hyperphosphatemia, CKD). In the absence of secondary hyperparathyroidism, patients with an elevated serum PTH but normal serum calcium have normocalcemic hyperparathyroidism. Such individuals require monitoring since hypercalcemia develops in about 19% of patients over 3 years of follow-up.
Genetic testing is recommended for patients with documented primary hyperparathyroidism who are younger than age 40, have a family history of hyperparathyroidism, or have multiglandular disease.
C. Imaging
Parathyroid imaging is not necessary for the diagnosis of hyperparathyroidism but is performed for most patients prior to parathyroid surgery. The visualization of an apparent parathyroid adenoma helps secure the diagnosis when there is occasional diagnostic difficulty and often allows for minimally invasive surgery.
Ultrasound should be performed with a high-resolution transducer (5-15 MHz) and should scan the neck from the mandible to the superior mediastinum in an effort to locate ectopic parathyroid adenomas. Ultrasound has a sensitivity of 79% for single adenomas but only 35% for multiglandular disease. Ultrasound is useful for the surgeon to localize parathyroid adenomas while also assessing the vocal folds. Enlarged parathyroids appear as ovoid, homogeneous, hypoechoic structures that are 0.8-1.5 cm in length and less compressible than surrounding tissue. Doppler imaging assists in distinguishing parathyroid adenomas from other structures.
Sestamibi scintigraphy with 99m Tc-sestamibi is most useful for localizing parathyroid adenomas. However, false-positive scans are common, caused by thyroid nodules, thyroiditis, or cervical lymphadenopathy. Sestamibi-SPECT imaging improves sensitivity for single parathyroid adenomas. Dual-phase imaging at 10-15 minutes, in addition to the usual imaging at 90-180 minutes, can identify the occasional parathyroid adenoma with rapid sestamibi washout that is not visible with later imaging. Sestamibi-iodine subtraction scanning and neck ultrasonography can locate parathyroid adenomas preoperatively in an effort to improve the outcome and limit the invasiveness of neck surgery. However, preoperative imaging has been used with only modest success. (See Surgery.) Small benign thyroid nodules are discovered incidentally in nearly 50% of patients with hyperparathyroidism who have imaging with ultrasound or MRI.
18 F-flurocholine PET/MRI is a useful scan for patients with primary hyperparathyroidism and negative or discordant localization imaging on neck ultrasound and sestamibi scanning. This scan correctly localizes a parathyroid adenoma in about 75% of cases.
Conventional CT and MRI imaging are not usually required prior to a first neck surgery for hyperparathyroidism. However, a four-dimensional CT (4D-CT), with the fourth dimension referring to time, captures the rapid uptake and washout of contrast from parathyroid adenomas; is useful for preoperative imaging when ultrasonography and sestamibi scans are negative. It can also be helpful for patients who have had prior neck surgery and for those with ectopic parathyroid glands. In such patients, 4D-CT has a sensitivity of 88%, but a lower specificity. MRI may also be useful for repeat neck operations and when ectopic parathyroid glands are suspected. MRI shows better soft tissue contrast than CT.
Noncontrast CT scanning of the kidneys in patients with hyperparathyroidism can visualize calcium-containing stones. However, for patients with mild and apparently asymptomatic hyperparathyroidism, only about 5% are found to have unsuspected nephrolithiasis.
Bone density measurements by dual energy x-ray absorptiometry (DXA) are helpful in determining the amount of cortical bone loss in patients with hyperparathyroidism. DXA should include three areas: distal radius (cortical), hip (cortical and trabecular), and lumbar vertebrae (trabecular). Vertebral bone density is usually not diminished in hyperparathyroidism.
Bone radiographs are usually normal and are not required to make the diagnosis of hyperparathyroidism. There may be demineralization, subperiosteal resorption of bone (especially in the radial aspects of the fingers), or loss of the lamina dura of the teeth. There may be cysts throughout the skeleton, mottling of the skull ("salt-and-pepper appearance"), or pathologic fractures. Articular cartilage calcification (chondrocalcinosis) is sometimes found.
Patients with renal osteodystrophy may have ectopic calcifications around joints or in soft tissue. Such patients may exhibit radiographic changes of osteopenia, osteitis fibrosa cystica, or osteosclerosis, alone or in combination. Osteosclerosis of the vertebral bodies is known as "rugger jersey spine."
Complications
Pathologic long bone fractures are a complication of hyperparathyroidism. UTI due to stone and obstruction may lead to kidney disease and uremia. If the serum calcium level rises rapidly, clouding of sensorium, kidney disease, and rapid precipitation of calcium throughout the soft tissues may occur (calciphylaxis). Peptic ulcer and pancreatitis may be intractable before surgery. In patients with MEN type 1 insulinomas, gastrinomas or pituitary tumors may be associated. Pseudogout may complicate hyperparathyroidism both before and after surgical removal of tumors. Hypercalcemia during gestation produces neonatal hypocalcemia.
In tertiary hyperparathyroidism due to CKD, high serum calcium and phosphate levels may cause calciphylaxis; calcification of arteries can result in painful ischemic necrosis of skin and gangrene, cardiac arrhythmias, and respiratory failure. The actual serum levels of calcium and phosphate have not correlated well with calciphylaxis, but a calcium (mg/dL) × phosphate (mg/dL) product over 70 is usually present.
Differential Diagnosis
Artifactual hypercalcemia is common, so a confirmatory serum calcium level should be drawn after an overnight fast along with a serum protein, albumin, and triglyceride while ensuring that the patient is well-hydrated. Hypercalcemia may be due to high serum protein concentrations; in the presence of very high or low serum albumin concentrations, an adjusted serum calcium or a serum ionized calcium is more dependable than the total serum calcium concentration. Hypercalcemia may also be seen with dehydration. Spurious elevations in serum calcium have also been reported with severe hypertriglyceridemia, when the calcium assay uses spectrophotometry. Artefactual high serum PTH can also occur with assay interference by human anti-mouse antibodies in certain assays. Unexpectedly high serum PTH levels should be repeated with a different assay.
Hypercalcemia of malignancy occurs most frequently with breast, lung, pancreatic, uterine, and renal cell carcinoma, and paraganglioma. Osteolytic bone metastases may or may not be present. Most of these tumors secrete PTH-related protein (PTHrP) that has structural homologies to PTH and causes bone resorption and hypercalcemia similar to those caused by PTH. Serum PTH levels are low or low-normal while serum PTHrP levels are elevated; phosphate is often low. Other tumors can secrete excessive 1,25 (OH)2 vitamin D3, particularly lymphoproliferative and ovarian malignancies. Plasma cell myeloma causes hypercalcemia in older individuals. Plasma cell myeloma causes kidney dysfunction; resultant increased levels of carboxyl terminal PTH may cause it to be confused with hyperparathyroidism if a carboxyl terminal PTH assay is used. Other hematologic cancers associated with hypercalcemia include monocytic leukemia, T-cell leukemia and lymphoma, and Burkitt lymphoma. The clinical features of malignant hypercalcemia can closely simulate hyperparathyroidism.
Pseudohyperparathyroidism of pregnancy presents with hypercalcemia during pregnancy. It is caused by hypersensitivity of the breasts to PRL. The breasts become abnormally enlarged and secrete excessive amounts of PTHrP that causes hypercalcemia. Treatment with dopamine agonists reverses the hypercalcemia.
Sarcoidosis and other granulomatous disorders, such as tuberculosis, berylliosis, histoplasmosis, coccidioidomycosis, leprosy, and foreign-body granuloma, can cause hypercalcemia. Sarcoid granulomas can secrete PTHrP, but granulomas secrete 1,25(OH)2D3 and serum levels of 1,25(OH)2D3 are usually elevated in the presence of hypercalcemia. However, in hypercalcemia with disseminated coccidiomycosis, serum 1,25(OH)2D3 levels may not be elevated. Serum PTH levels are usually low.
Dietary factors can cause hypercalcemia. Excessive calcium, vitamin D, or vitamin A ingestion can cause hypercalcemia, especially in patients who concurrently take thiazide diuretics, which reduce urinary calcium loss. Hypercalcemia is reversible following withdrawal of calcium and vitamin D supplements. If hypercalcemia persists, the possibility of associated hyperparathyroidism should be considered. In vitamin D intoxication, hypercalcemia may persist for several weeks. Serum levels of 25-hydroxycholecalciferol (25[OH]D3) are helpful to confirm the diagnosis. A brief course of corticosteroid therapy may be necessary if hypercalcemia is severe. A severely carbohydrate-restricted ketogenic diet can also cause hypercalcemia with a low serum PTH.
Familial hypocalciuric hypercalcemia (FHH) is an uncommon autosomal dominant inherited disorder (prevalence: 13 per million persons). Three different loss-of-function germline mutations have been recognized as causative. FHH1, the most common cause of FHH, is caused by an inactivating germline mutation in the CASR gene that encodes the extracellular CaSR. Less commonly, an inactivating mutation in GNA11 causes FHH2 and a mutation in AP2S1 causes FHH3; these two genes encode downstream molecules involved with the intracellular calcium sensing pathway within the parathyroid cell. Serum calcium levels tend to be higher in FHH3 compared to FHH1. Reduced function of the CaSR causes the parathyroid glands to falsely "sense" hypocalcemia and inappropriately release excessive amounts of PTH. The renal tubule CaSRs are also affected, causing hypocalciuria.
FHH was previously termed "benign FHH" and thought to be asymptomatic. FHH can sometimes present with neonatal severe primary hyperparathyroidism. Adults with hypercalcemia due to FHH are either asymptomatic or have nonspecific complaints such as fatigue, weakness, or cognitive issues. Recurrent pancreatitis can occur.
FHH is characterized by a mildly elevated serum calcium that is usually below 11.0 mg/dL (2.75 mmol/L) and a low urine calcium excretion that is usually less than 50 mg/24 hours (13 mmol/24 hours). Serum PTH levels are usually normal or minimally elevated. Serum phosphate levels are normal. About 4% of patients with true hyperparathyroidism can have a low urine calcium (below 100 mg/day). Therefore, FHH is confirmed with genetic testing for FHH gene mutations. These patients do not normalize their hypercalcemia after subtotal parathyroid removal and should not be subjected to surgery. Cinacalcet, a calcimimetic, may be helpful.
Prolonged immobilization at bed rest commonly causes hypercalcemia, particularly in adolescents, critically ill patients, and patients with extensive Paget disease of bone. Hypercalcemia develops in about one-third of acutely ill patients being treated in ICUs, particularly patients with AKI. Serum calcium elevations are typically mild but may reach 15 mg/dL (3.75 mmol/L). Serum PTH levels are usually slightly elevated, consistent with mild hyperparathyroidism but may be suppressed or normal.
Rare causes of hypercalcemia include untreated adrenal insufficiency. This is partly due to disinhibition of calcium uptake by the renal tubule and gut. Additionally, Addison disease can cause dehydration and hyperproteinemia, resulting in higher levels of nonionized calcium. Modest hypercalcemia is occasionally seen in patients taking thiazide diuretics or lithium; the PTH level may be inappropriately nonsuppressed with hypercalcemia. Hyperthyroidism causes increased turnover of bone and occasional hypercalcemia. Bisphosphonates can increase serum calcium in 20% and serum PTH becomes high in 10%, mimicking hyperparathyroidism. Other causes of hypercalcemia are shown in Table 23-7.
Treatment
A. "Asymptomatic" Primary Hyperparathyroidism
Normocalcemic or mild hyperparathyroidism should be considered "asymptomatic" only after obtaining a detailed patient medical history. Many patients may not recognize subtle manifestations, such as cognitive slowing, having become accustomed to them over years. It is important to assess blood pressure, serum BUN and creatinine, and to determine the presence of nephrolithiasis or nephrocalcinosis by radiography, ultrasonography, or CT of the kidneys. Truly asymptomatic patients may be closely monitored and advised to keep active, avoid immobilization, and drink adequate fluids. For postmenopausal women with hyperparathyroidism, estrogen replacement therapy reduces serum calcium by an average of 0.75 mg/dL (0.19 mmol/L) and slightly improves bone density. For patients with hypercalciuria (more than 400 mg daily) or calcium nephrolithiasis, hydrochlorothiazide may be used in doses of 12.5-25 mg daily to reduce calciuria; however, serum calcium must be monitored carefully. Parathyroidectomy does not improve the bone density of patients with osteoporosis who have normocalcemia or normocalcemic hyperparathyroidism.
Affected patients should avoid large doses of thiazide diuretics, vitamin A, and calcium-containing antacids or supplements. Serum calcium and albumin are checked at least twice yearly, kidney function and urine calcium once yearly, and three-site bone density (lumbar vertebrae, hip, and distal radius) every 2 years. Rising serum calcium should prompt further evaluation and determination of serum PTH levels.
If it is not clear whether a patient with primary hyperparathyroid is symptomatic, it is reasonable to consider a trial of medical therapy with cinacalcet.
B. Medical Measures
1. Fluids
Hypercalcemia is treated with a large fluid intake unless contraindicated. Severe hypercalcemia requires hospitalization and intensive hydration with intravenous saline. (See Part 23.)
2. Casr Activators
Cinacalcet is a calcimimetic agent that binds to sites of the parathyroid glands' extracellular CaSRs to increase the glands' affinity for extracellular calcium, thereby decreasing PTH secretion. Cinacalcet may be used as the initial therapy for patients with hyperparathyroidism or for failed surgical parathyroidectomy. For primary hyperparathyroidism with mild hypercalcemia, begin cinacalcet (15 mg orally [one-half of a 30-mg tablet]) and monitor the serum calcium weekly; increase the dose every 2 weeks if hypercalcemia persists until the patient becomes normocalcemic, which is successful in about 65% of sporadic cases and 80% of familial cases. Patients with parathyroid carcinoma and severe hypercalcemia are treated with cinacalcet in addition to the bisphosphonate, zoledronic acid. For parathyroid cancer, cinacalcet is administered in doses of 30 mg orally twice daily, increased progressively to 60 mg twice daily, then 90 mg twice daily to a maximum of 90 mg every 6-8 hours. Cinacalcet is usually well tolerated but may cause nausea and vomiting (11%), myalgia, or malaise. Cinacalcet does not usually correct hypercalciuria. Hypocalcemia has occurred, even at 30 mg/day.
About 50% of azotemic patients with secondary or tertiary hyperparathyroidism have hypercalcemia that is resistant to vitamin D analogs. In such cases, surgical parathyroidectomy offers lower mortality rates than cinacalcet. But for patients in whom surgery is contraindicated, or as an interim therapy, cinacalcet may be given in doses of 30 mg orally daily to a maximum of 250 mg daily, with dosage adjustments to keep the serum PTH in the range of 150-300 pg/mL (15.8-31.6 pmol/L). Etelcalcetide also activates the parathyroid glands' CaSR and reduces hypercalcemia in dialysis patients; it is given intravenously at the end of hemodialysis sessions, thereby avoiding the GI side effects of cinacalcet.
3. Bisphosphonates
Intravenous bisphosphonates are potent inhibitors of bone resorption and can temporarily treat the hypercalcemia of hyperparathyroidism. Following intravenous administration, pamidronate (30-90 mg) and zoledronic acid (5 mg) cause a gradual decline in serum calcium over several days that may last for weeks to months. Intravenous bisphosphonates are used generally for patients with severe hyperparathyroidism in preparation for surgery. Oral bisphosphonates, such as alendronate, are not effective for treating the hypercalcemia or hypercalciuria of hyperparathyroidism. However, oral alendronate has been shown to improve BMD in the trabecular bone of the lumbar spine and hip (not distal radius) and may be used for asymptomatic patients with hyperparathyroidism who have a low BMD. It may also be combined with cinacalcet for the medical treatment of osteoporosis in patients with persistent hyperparathyroidism.
4. Denosumab
For patients with severe hypercalcemia due to parathyroid carcinoma, denosumab 120 mcg subcutaneously monthly may be effective. However, high-dose denosumab increases the risk of jaw osteonecrosis and serious infections.
5. Vitamin D and Vitamin D Analogs
6. Other Measures
Estrogen replacement reduces hypercalcemia slightly in postmenopausal women with hyperparathyroidism. Similarly, oral raloxifene (60 mg/day) may be given to postmenopausal women with hyperparathyroidism; it reduces serum calcium an average of 0.4 mg/dL (0.1 mmol/L), while having an anti-estrogenic effect on breast tissue. Beta-blockers, such as propranolol, may also be useful for preventing the adverse cardiac effects of hypercalcemia. Parathyroid carcinoma metastases may be treated with radiofrequency ablation or arterial embolization.
C. Surgical Parathyroidectomy
Parathyroidectomy is recommended for patients with hyperparathyroidism who are symptomatic or who have nephrolithiasis or parathyroid bone disease. During pregnancy, parathyroidectomy is performed in the second trimester for women who are symptomatic or have a serum calcium above 11 mg/dL (2.75 mmol/L).
Some patients with seemingly asymptomatic hyperparathyroidism may be surgical candidates for other reasons such as (1) serum calcium 1 mg/dL (0.25 mmol/L) above the upper limit of normal, (2) urine calcium excretion greater than 400 mg/day (10 mmol/day), (3) eGFR less than 60 mL/min/1.73 m2 , (4) nephrolithiasis or nephrocalcinosis, (5) cortical bone density (wrist, hip, or distal radius) indicating osteoporosis (T score below -2.5) or previous fragility bone fracture, (6) relative youth (under age 50 years), (7) difficulty ensuring medical follow-up, or (8) pregnancy.
Surgery for patients with "asymptomatic" hyperparathyroidism may improve cortical BMD and confer modest benefits in social and emotional well-being and overall quality of life in comparison to similar patients being monitored without surgery. Cognitive function may benefit with improvements in nonverbal abstraction and memory.
Preoperative parathyroid imaging has been used in an attempt to allow unilateral minimally invasive neck surgery (see Imaging, above). The reported success rates vary considerably. Even in patients with concordant sestamibi and ultrasound scans, and an intraoperative PTH drop of more than 50%, hyperparathyroidism may persist postoperatively in up to 15% of patients.
Without preoperative localization studies, bilateral neck exploration is usually advisable for the following: (1) patients with a family history of hyperparathyroidism, (2) patients with a personal or family history of MEN, and (3) patients wanting an optimal chance of success with a single surgery. Patients undergoing unilateral neck exploration can have the incision widened for bilateral neck exploration if two abnormal glands are found or if the serum quick PTH level falls by less than 63% within 10 minutes of the parathyroid resection. Parathyroid glands are often supernumerary (five or more) or ectopic (eg, intrathyroidal, carotid sheath, mediastinum).
For patients with MEN type 1, the optimal surgical management is subtotal parathyroidectomy. Recurrent hyperparathyroidism develops in 18%, and the rate of postoperative hypoparathyroidism is high. In such cases, a parathyroid gland may be transplanted into a neck muscle, from which it may be easily removed if hyperparathyroidism persists. About 30% of patients with successful parathyroid surgery continue to have an elevated serum PTH postoperatively, despite normal serum calcium levels; this is sometimes due to vitamin D deficiency.
Intraoperative quick serum PTH monitoring is helpful. Serum PTH levels that fall greater than 63% within 10 minutes of a parathyroid adenoma resection indicates a high likelihood that the surgery will be curative.
Intraoperative near-infrared autofluorescence spectroscopy imaging may improve intraoperative localization of parathyroid glands in primary hyperparathyroidism, particularly when preoperative localization studies have been negative or discrepant. The parathyroid glands have intrinsic near-infrared fluorescence that can be detected with a specialized camera. However, operating lights must be turned off during image capture.
Parathyroid hyperplasia is commonly seen with secondary or tertiary hyperparathyroidism associated with uremia that is resistant to vitamin D analogues. Parathyroidectomy offers lower mortality rates, compared with cinacalcet. When surgery is performed, a subtotal parathyroidectomy is optimal; three and one-half glands are usually removed, and a metal clip is left to mark the location of residual parathyroid tissue.
Parathyroid carcinoma surgery consists of either parathyroidectomy or en bloc resection of the tumor and ipsilateral thyroid lobe with care to avoid rupturing the tumor capsule. If the surgical margins are not clear of tumor, postoperative neck radiation therapy may be given. Local and distant metastases may be debulked or irradiated. Preoperative MRI scanning is required to delineate the tumor. Zoledronic acid or denosumab is given preoperatively. Severe hypercalcemia requires multiple medical measures, including hydration, furosemide, cinacalcet, zoledronic acid, or denosumab. Radiation therapy can be given for localized tumor. Osseous metastases must be distinguished from benign brown tumors caused by hyperparathyroidism; biopsy may be required. Chemotherapy has been ineffective for patients with distant metastases. Immunotherapy with anti-hPTH monoclonal antibodies is a treatment option.
Complications-Serum PTH levels fall below normal in 70% of patients within hours after successful surgery, commonly causing hypocalcemic paresthesias or even tetany. Hypocalcemia tends to occur the evening after surgery or on the next day. Frequent postoperative monitoring of serum calcium (or serum calcium plus albumin) is advisable beginning the evening after surgery. Once hypercalcemia has resolved, liquid or chewable calcium carbonate is given orally to reduce the likelihood of hypocalcemia. Symptomatic hypocalcemia is treated with larger doses of calcium; calcitriol (0.25-1 mcg daily orally) may be added, with the dosage depending on symptom severity. Magnesium salts are sometimes required postoperatively, since adequate magnesium is required for functional recovery of the remaining suppressed parathyroid glands.
In about 12% of patients having successful parathyroid surgery, PTH levels rise above normal (while serum calcium is normal or low) by 1 week postoperatively. This secondary hyperparathyroidism is probably due to "hungry bones" and is treated with calcium and vitamin D preparations. Such therapy is usually needed only for 3-6 months but is required long term by some patients.
Hyperthyroidism commonly occurs immediately following parathyroid surgery. It is caused by release of stored thyroid hormone during surgical manipulation of the thyroid. In symptomatic patients, short-term treatment with propranolol may be required for several days.
Prognosis
Patients with symptomatic hyperparathyroidism usually experience worsening disease (eg, nephrolithiasis) unless they have treatment. Conversely, the majority of completely asymptomatic patients with a serum calcium below 11.0 mg/dL (2.75 mmol/L) remain stable with follow-up. However, worsening hypercalcemia, hypercalciuria, and reductions in cortical BMD develop in about one-third of asymptomatic patients. Therefore, asymptomatic patients must be monitored carefully and treated with oral hydration and mobilization.
Surgical removal of apparently single sporadic parathyroid adenomas is successful in 94%. Patients with MEN 1 undergoing subtotal parathyroidectomy may experience long remissions, but hyperparathyroidism frequently recurs. Despite treatment for hyperparathyroidism, hypertension is usually not reversed and patients remain at increased risk for all-cause mortality, CVD, renal calculi, and kidney dysfunction.
Spontaneous cure due to necrosis of the tumor is exceedingly rare. The bones, in spite of severe cyst formation, deformity, and fracture, will heal if hyperparathyroidism is successfully treated. The presence of pancreatitis increases the mortality rate. Acute pancreatitis usually resolves with correction of hypercalcemia, whereas subacute or chronic pancreatitis tends to persist. Kidney damage may progress even after removal of a parathyroid adenoma.
Parathyroid carcinoma is associated with 5- and 10-year survival rates of 78% and 49%, respectively. A better prognosis is associated with clear surgical margins and no detectable metastases postoperatively. Conversely, positive surgical margins or metastases predict a very poor 5-year survival. The prognosis is also poorer for nonfunctioning parathyroid carcinoma and those tumors that carry a CDC73 mutation, loss of fibromin, or loss of CaSR expression. Repeat surgical debulking procedures may improve survival. Aggressive medical management can also prolong life.
When to Admit
Patients with severe hypercalcemia for intravenous hydration.
Appleman-DijkstraNMet al. Approach to the patient: management of parathyroid disease in pregnancy. J Clin Endocrinol Metab. 2023;108:1505. [PMID: 36546344] CarpenterTOet al. Case 32-2021: a 14-year-old girl with swelling of the jaw and hypercalcemia. N Engl J Med. 2021;385:1604. [PMID: 34670047] ChandranMet al. The efficacy and safety of cinacalcet in primary hyperparathyroidism: a systematic review and meta-analysis of randomized controlled trials and cohort studies. Rev Endocr Metab Disord. 2022;23:485. [PMID: 35041148] DavisCet al. Hyperparathyroidism in pregnancy. BMJ Case Rep. 2020;13:e232653. [PMID: 32066577] KanisJAet al. Primary hyperparathyroidism and fracture probability. Osteoporos Int. 2023;34:489. [PMID: 36525071] KomabaHet al. Parathyroidectomy vs cinacalcet among patients undergoing hemodialysis. J Clin Endocrinol Metab. 2022;107:2016. [PMID: 35277957] LeereJSet al. Denosumab and cinacalcet for primary hyperparathyroidism (DENOCINA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2020;8:407. [PMID: 32333877] McInerneyNJet al. Parathyroid carcinoma: current management and outcomes - a systematic review. Am J Otolaryngol. 2023;44:103843. [PMID: 36989753] MotlaghzadehYet al. Rare causes of hypercalcemia: 2021 update. J Clin Endocrinol Metab. 2021;106:3113. [PMID: 34240162] RodrigoJPet al. Parathyroid cancer: an update. Cancer Treat Rev. 2020;86:102012. [PMID: 32247225] SarquisMet al. Long-term remission of disseminated parathyroid cancer following immunotherapy. Endocrine. 2020;67:204. [PMID: 31782130] SlatteryLet al. Contemporary management of primary hyperparathyroidism. Surg Clin North Am. 2022;10:251. [PMID: 35344696] ZamanMet al. Long-term recurrence rates after surgery in primary hyperparathyroidism. J Clin Endocrinol Metab. 2023;108:3022. [PMID: 37279502] ZareiAet al. Multimodality imaging in primary hyperparathyroidism. Clin Radiol. 2022;77:e401. [PMID: 35393101] ZhuCYet al. Diagnosis and management of primary hyperparathyroidism. JAMA. 2020;323:1186. [PMID: 32031566] |