Description
- The balance between myocardial oxygen supply and demand is critical to the proper function of the heart. If myocardial oxygen demands are not met, myocardial ischemia, infarction, arrhythmias, or death may result.
- Oxygen demand is not equivalent to oxygen consumption. Demand is related to need. Consumption is the actual amount of oxygen used per unit time.
- The myocardium uses oxygen primarily for effective contraction. Requirements for basal metabolism comprise only 10–20% of total O2 consumption. Requirements of the electrical conduction system are even less.
- The major determinants of myocardial oxygen demand are:
- Heart rate
- Contractility
- Myocardial (systolic) wall tension
- Myocardial oxygen consumption (VO2): Large amounts of ATP are needed for the proper function of the myocardium. Aerobic metabolism (most efficient source) of fatty acids is the primary mechanism by which ATP is regenerated at rest.
- Oxygen extraction: The myocardium is a tightly regulated tissue which excels in its ability to extract oxygen from the blood that enters the coronary arteries. In the non-stressed state, the myocardium extracts 70–75% of the oxygen from the red blood cells/hemoglobin that flow through the coronary arteries. Venous saturation thus equals ~25–30%.
- Coronary blood supply is comprised of coronary blood flow and blood oxygen carrying capacity. Furthermore, because coronary oxygen extraction is near maximal under resting conditions, the primary mechanism to meet increased oxygen demand is through enhanced delivery; thus, it is a dynamic process that is modulated by multiple parameters.
- Autoregulation is the intrinsic mechanism to maintain a constant blood flow over a range of perfusion pressures (60–160 mm Hg); changes in pressure are met by reciprocal changes in resistance. Local metabolic and myogenic factors appear to play a role. Myogenic factors describe the response of smooth muscles to the shear forces of perfusion pressure. Metabolic factors (e.g., oxygen and carbon dioxide tension and adenosine) affect vascular smooth muscle tone as their concentrations vary. In other words, an increase in the PaCO2 would stimulate vasodilation and "washout."
- Humoral factors include circulating agents such as angiotensin II, serotonin, thromboxane, prostacyclin, and bradykinin that effect coronary resistance.
- Neural control describes autonomic innervation such as alpha, beta, and parasympathetic activity.
- Diastolic time: The left ventricle's systolic pressures exceed the coronary artery's diastolic perfusion pressure; thus extravascular compressive forces prevent perfusion during systole.
- Blood oxygen–carrying capacity is the sum of oxygen bound to hemoglobin and dissolved in blood. It is primarily determined by the hemoglobin level (capable of binding 1.39 mL of oxygen per gram); dissolved oxygen is poorly soluble in blood (0.003), thus, its contribution is minimal. This serves as the physiologic basis for blood transfusions.
- Oxygen demand: Heart rate has the greatest effect on myocardial work and oxygen demand.
- Heart rate: for every heartbeat, the myocardium undergoes electrical depolarization and repolarization, generates contractility, ejects blood against the wall tension (preload or volume work and afterload or pressure work), and undergoes relaxation. Note: Tachycardia will decrease the diastolic perfusion time which primarily affects the left ventricle's blood supply.
- Cardiac contractility or inotropy is the intrinsic ability of the cardiomyocyte to shorten from its individual resting fiber length; it is independent of preload and afterload. Contraction results from myosin and actin filament binding and is dependent upon intracellular calcium ion concentration. Increases in sympathetic tone or catecholamine state, calcium levels, heart rate, as well as inotropic drugs (beta agonists, calcium, glucagon, phosphodiesterase inhibitors) have a positive effect on contractility.
- Myocardial wall tension: The law of LaPlace states that wall tension is directly proportional to the chamber radius and pressure; it is inversely proportional to the wall thickness.
- Radius is primarily determined by preload (changes in blood volume or chamber size).
- Pressure is primarily determined by the afterload which is a function of systemic vascular resistance, the aortic valve, and blood viscosity.
- Myocardial wall thickness is responsible for long-axis function secondary to endocardial and midwall fractional shortening. However, wall thickness is not responsible for short-axis function.
- Tension is dynamic (aside from wall thickness) and can vary with each heartbeat and throughout the contraction (chamber size decreases with ejection).
- Coronary arteries: The presence of a stenotic lesion results in a pressure drop that is proportional to the fourth power of the radius, the length of the plaque, and the magnitude of flow.
- The subendocardium (inner one-third to one-fourth of the myocardium) is most susceptible to the effects of CAD or to a reduction in perfusion pressure. This is secondary to:
- Adjacent intraventricular pressures (left ventricular end-diastolic pressure)
- Limited maximal vasodilator response
- Blood flow only occurs during diastole; during systole, extravascular compressive forces (mechanical forces that compress coronary vasculature) are greatest
Physiology/Pathophysiology
- Heart rate is increased by:
- Cardiac contractility is increased by:
- Catecholamines
- Increased heart rate: The Treppe or Bowditch effect describes an autoregulatory method by which myocardial contractility increases with an increase in heart rate. This mechanism is believed to result from an inability of the sodium–potassium ATPase (moves sodium extracellularly) to keep up with the sodium–calcium exchanger (moves sodium intracellularly) during tachycardia. This results in increased intracellular levels of calcium.
- Myocardial wall tension is increased by:
- Increased ventricular diameter (volume overload, impaired ejection fraction, dilated cardiomyopathy).
- Increased aortic pressure: Hypertension, aortic stenosis, and hemoconcentration increase the pressure that needs to be generated in order to eject blood from the left ventricle.
- Induction of anesthesia
- Laryngoscopy and airway instrumentation can result in excessive catecholamine release. The goal is to pre-empt with opioid or anti-hypertensive administration, decrease laryngoscopy time, ensure an adequate depth of anesthesia (and time for onset), and be prepared to treat hemodynamic perturbances (esmolol, nitroglycerin, additional anesthetic agents such as propofol or volatile agents).
- Maintenance of anesthesia
- Pain and awareness under anesthesia can result in tachycardia; ensure an adequate depth of analgesia and anesthesia. This may become challenging in the hypotensive patient. Consider the use of nitrous oxide and decrease of volatile agent, volume administration, or phenylephrine infusion to maintain adequate mean arterial pressures.
- Perioperative beta-blockade may be titrated to a heart rate of 50–80 beats/min or bolused during catecholaminergic states.
- Changes to desflurane concentrations can result in sympathetic discharge and tachycardia.
- Normothermia may be maintained with blankets or warming devices (convective blankets, warming fluids, etc.)
- Optimize volume status: Hypovolemia can result in a reflex tachycardia, whereas hypervolemia increases the myocardial chamber radius (preload).
- Postoperative/PACU care
- Provide adequate pain control: A multimodal approach may include anti-inflammatory medications, regional nerve blocks, and opioids, as appropriate.
- Optimize volume status: The postoperative period may be marked by fluid flux between the intracellular and extracellular compartments as well as continued bleeding.
- Treat postoperative shivering (warming techniques, meperidine, clonidine).
- Ensure adequate oxygenation: Residual sedatives or narcotics can decrease the functional residual capacity and result in hypoxia (increased sympathetic state). Consider nasal cannula, facemask, deep breathing, coughing or CPAP/BIPAP when indicated.
- MVO2 = CBF × (CaO2 – CvO2); where MVO2 is mixed venous oxygen saturation, CBF is cerebral blood flow, CaO2 is the arterial blood oxygen content, and CvO2 is the venous blood oxygen content
- A-VO2 = (CaO2 – CvO2) is the arterial-venous oxygen content difference (mL O2/mL blood)
- CO = O2 consumption/A-VO2 difference
- Law of LaPlace: T =
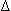 Pr/2h, where T is tension,
Pr/2h, where T is tension, 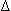 P is change in pressure, r is radius, and h is myocardial wall thickness
P is change in pressure, r is radius, and h is myocardial wall thickness