Description
- Left ventricular (LV) afterload is the force against which the heart must work to generate pump work (eject blood out of the heart into the aorta).
- In the purest form, afterload is defined as wall stress, which are the forces opposed to ventricular fiber shortening. Afterload or wall stress provides the most accurate indication of cardiac energy expenditure. However, clinically true afterload or wall stress cannot be measured.
- Ventricular afterload is comprised of a static and dynamic component; elevation in either component may result in increased afterload.
- Static component of the LV is comprised of the systemic vascular resistance (SVR) or outflow of blood into resistance arterioles. Under normal physiologic circumstances and in healthy (compliant) vasculature, the SVR is the main determinant of LV afterload.
- Small arteries and arterioles are referred to as resistance vessels since they are the principal site of SVR. The autonomic nervous system provides constant regulation on a short time scale (seconds to minutes).
- SVR; Normal 800–1400 dyne-s/cm5. SVR can be calculated from MAP, CVP, CO; SVR = [(MAP – CVP) × 80]/CO. The pressures are measured from the "beginning" of the circuit to the "end" of the circuit. for example, if the CO is 5 L/min, the BP is 120/80 mm Hg (MAP is 93.3 mm Hg), and the CVP is 12 mm Hg, then SVR = [(93.3–12) × 80]/5 = 1396 dyne-s/cm5.
- The static component of the right ventricle (RV) is composed of the pulmonary vascular resistance (PVR); normal 80–120 dyne-s/cm5. The blood flow (CO) through the left and right sides of the heart are equal; however, the afterload for the RV is significantly less. The main component of RV afterload is PVR, which is around 10 times lower than SVR. The formula to calculate is: PVR = [(Mean PAP – wedge pressure) × 80]/CO. The pressures are measured from the "beginning" of the circuit to the "end" of the circuit. for example, if the PA pressures is 25/10 mm Hg (Mean PAP is 15 mm Hg), the wedge pressure is 8 mm Hg, and CO is 5 L/min, then PVR = [(15–8) × 80]/5 = 112 dyne-s/cm5.
- Dynamic pulsatile component: Consists of 2 phasic elements; one is the compliance-related forward pulse wave and the second is the reflected pulse wave. The pulsatile component contributes significantly to total LV afterload in stiff vessels. Central arterial vessels are the principal site of the pulsatile component.
- Static component of the LV is comprised of the systemic vascular resistance (SVR) or outflow of blood into resistance arterioles. Under normal physiologic circumstances and in healthy (compliant) vasculature, the SVR is the main determinant of LV afterload.
- Despite the clinical use of SVR to determine LV afterload, it is best measured as systolic wall stress. Stress (or tension) is defined as the force, or load, per unit cross-sectional area. Wall stress/tension is the product of transmural pressure and chamber radius divided by the wall thickness. for the LV: S = PR/2h; where S is the LV wall stress, P is the LV cavity pressure, R is the radius of the LV, and h is the myocardial thickness (also known as LaPlace's Law).
- LV cavity pressure
- During systole, the LV generates pressure. That pressure should be higher than pressure seen by the LV in the ascending aorta at each point in time during ejection to promote forward blood flow. Pressure in the ascending aorta will depend on the peripheral resistance, compliance of the arterial tree, and contributions of wave reflections.
- Aortic valve: Any obstruction to flow from the LV cavity to the ascending aorta will cause additional resistance and a necessary increase in the LV cavity pressure to overcome it. Clinically, the most common causes are aortic stenosis and hypertrophic obstructive cardiomyopathy which can cause up to 100 mm Hg extra LV pressure over what is needed to pump blood into aorta.
- Blood viscosity contributes to the resistance to blood flow, such that increased viscosity leads to increased resistance. The blood viscosity depends, for the most part, on the hematocrit.
- Radius: Wall stress (afterload) is dependent on the geometry of the LV itself. During systole, the ventricular wall thickens and the radius decreases as the ventricle pumps blood out (and reduces LV wall stress). Preload or LV end diastolic volume affects the initial radius and, hence, wall stress during isovolemic contraction.
- Myocardial thickness: In cases of pressure overload, and resultant increases in wall stress, the heart muscles compensate by becoming hypertrophic (wall stress is inversely related to wall thickness, thus myocardial hypertrophy decreases wall stress). Increased muscle mass requires less force per unit area.
- Wall stress or afterload varies over time since the LV pressure, radius, and wall thickness are continuously changing throughout the cardiac cycle. As a result, afterload can be calculated at the instant of aortic valve opening, at the end of ejection, or at any instant throughout systole. Clinical investigators use peak systolic stress, end-systolic stress, or mean systolic wall stress as indices of afterload.
- LV cavity pressure
- Determinants/regulation of vascular resistance (and hence afterload)
- Neurohumoral responses (long-term regulation, over days and weeks)
- Renin–angiotensin axis: Juxtaglomerular cells in the kidneys secrete renin directly into the blood. The secreted renin then converts angiotensinogen (released by the liver) to angiotensin I. Angiotensin I is subsequently converted to angiotensin II by the enzyme angiotensin-converting enzyme (produced by the lungs). Angiotensin II is a potent vasoactive peptide that causes blood vessels to constrict, resulting in increased SVR.
- Vasopressin (antidiuretic hormone) is secreted into the blood by the neurohypophysis. It activates specific vasopressin receptors on the VSMC and causes vasoconstriction.
- Nitric oxide (NO) is produced by endothelial cells, diffuses into the media, and relaxes the VSMC by activating cyclic guanylate cyclase. Hence, NO is called endothelium-derived relaxation factor. Smoking, hypercholesterolemia, oxidative stress, or limited physical exercise can result in endothelial dysfunction; consequently, there is a decrease in NO production (increase in SVR).
- Hydrogen sulfide has been recently recognized as endothelium-derived hyperpolarizing factor. Similar to nitric oxide, hydrogen sulfide causes relaxation of resistance arterioles, vasodilation, and decreases the SVR.
- Neurohumoral responses (long-term regulation, over days and weeks)
- The mean pressure decreases by only 1–2 mm Hg between the ascending aorta and peripheral arteries in compliant and healthy vasculature, indicating low resistance at this portion of the vascular tree.
- A major drop of pressure occurs over small arterioles and they constitute the major site of resistance.
Physiology/Pathophysiology
- Increased afterload:
- Aortic stenosis: The LV needs to generate increased force to overcome the resistance of the stenosed aortic valve and eject blood into the aorta. This results in increased LV wall tension and stress (increased afterload). The LV usually compensates with a hypertrophic response to return the wall stress to normal. The thickened LV, in conjunction with increased force, however, increases the myocardial oxygen demand (increased muscle mass working harder) and decreases the myocardial oxygen supply, especially to the endocardium. Blood flows from the epicardium into the endocardium; additionally, it takes more time for blood to flow across a thickened LV wall to reach the endocardium.
- Chronic hypertension: Essential hypertension in the young and middle-aged population is frequently characterized by increases in DBP. This is the result of vasoconstrictory responses predominating over vasodilatory responses at the level of the arterioles in the periphery. In the elderly, the predominant form of hypertension is systolic hypertension, while DBP is low to normal (the SVR in isolated systolic hypertension is usually normal or even decreased). However, there is a marked increase in vascular sclerosis, stiffness, and arterial impedance. The increased resistance is to the pulsatile (dynamic) component of blood flow. In either case, untreated or poorly controlled hypertension requires the heart to work harder, increases afterload, and often elicits a compensatory hypertrophic response in the LV to return wall stress to normal.
- Hemoconcentration: Analogous to the reservoir bag on the anesthesia machine; if the bag was filled with water or jelly as opposed to air, it would require more energy to compress or squeeze the bag.
- Increased LV radius: A dilated LV, as with cardiomyopathy or heart failure, causes increases in wall tension due to decreased myocardial thickness as well as increased radius ( = increase in afterload). This is analogous to the reservoir bag overfilled; more energy would be required to empty the bag, compared to a partially or normally filled bag.
- Cor pulmonale: Elevated PVR requires the RV to squeeze harder to generate more force to eject blood ( = increased afterload for the RV). Elevations in the PVR over prolonged time lead to compensatory RV hypertrophy to decrease wall tension and normalize the afterload.
- Decreased afterload
- Sepsis: Mostly due to loss of SVR
- Anaphylaxis: Mostly due to loss of SVR
- High-output cardiac failure: Due to arteriovenous shunts
- Anemia: Due to both a decrease in viscosity and lowered SVR (decreased BP)
- Hypovolemia: There is less blood to stretch the LV and, hence, less force is needed to eject blood. To counteract for reductions in the stroke volume (and maintain BP), the sympathetic tone is increased (increase in SVR and heart rate).
- Increased afterload
- Decreased afterload
- An increasing depth of anesthesia with either volatile agents or IV anesthetics (e.g., propofol) causes a decrease in sympathetic tone (vasorelaxation) with resultant decreases in the SVR and BP. Increased depth should be avoided in hypovolemia or hypotensive patients.
- Neuraxial techniques: Both spinal and epidural blocks interrupt conduction of nerve impulses through sympathetic fibers at the level of their spread. This leads to a decrease in sympathetic tone, SVR, and preload (pooling of blood in veins). To counteract this response, volume resuscitation and pressors may be administered as needed. In hypovolemic or hemorrhaging patients, neuraxial techniques should not be used; they block compensatory increases in SVR and heart rate.
- NO donors (nitroglycerin, nitroprusside) cause relaxation of both resistance arterioles and veins and, hence, a decrease in SVR and preload. They are used for tight BP control in procedures such as AAA and CEA.
- Inhaled NO is used to alleviate increased pulmonary artery pressures. It has minimal systemic effects.
- Calcium channel blockers cause arterial smooth muscle relaxation and decrease myocardial contractility.
- Phosphodiesterase inhibitors increase cardiac contractility while relaxing resistance arterioles, decreasing SVR, and causing hypotension. They are mostly used in heart failure, but may also require simultaneous administration of alpha-agonists to counteract pronounced hypotension.
- Positive end expiratory pressure (PEEP). LV dysfunction with pulmonary edema results in decreased pulmonary volume and compliance. Greater negative inspiratory pressures (and hence transmural pressures) are required to expand the lungs. PEEP can partially offset this.
- SVR = [(MAP – CVP) × 80]/CO; MAP = mean arterial pressure (mm Hg), CVP = central venous pressure (mm Hg), CO = cardiac output (liters/minute)
- PVR = [(mPAP – wedge pressure) × 80]/CO; mPAP = mean pulmonary artery pressure (mm Hg), CO = cardiac output (liters/minute)
- Tension = (
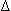 P × R)/H × 2;
P × R)/H × 2; 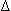 P = change in pressure, R = radius, H = wall thickness
P = change in pressure, R = radius, H = wall thickness