Description
- Spirometry reflects respiratory mechanics. It describes the relationship between maximal forceful exhaled volume (following maximal inspiration) and time.
- It is safe, inexpensive, and noninvasive.
- Maximal and reproducible patient effort is essential for the accuracy of the test.
- Spirometry, lung volumes, and diffusing capacity (DLCO) constitute pulmonary function testing (PFTs).
- Spirometry reflects a complex interaction of respiratory muscle strength, elastic properties of the chest wall and lungs, and airway resistance. for example, forced exhalation reflects the ability of the expiratory muscles to overcome the outward force of the thorax. Spirometry cannot measure airway resistance directly, rather it is estimated based on expiratory flows.
- Spirometry testing
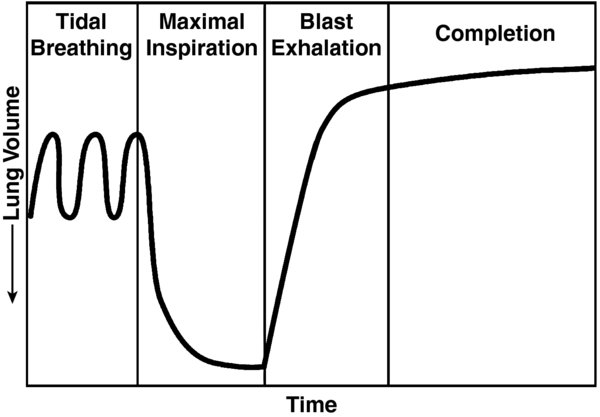
- During spirometry, patients are asked to breath normal tidal volumes (Fig.1), inhale maximally, and then exhale with maximal force (blast) and length of exhalation.
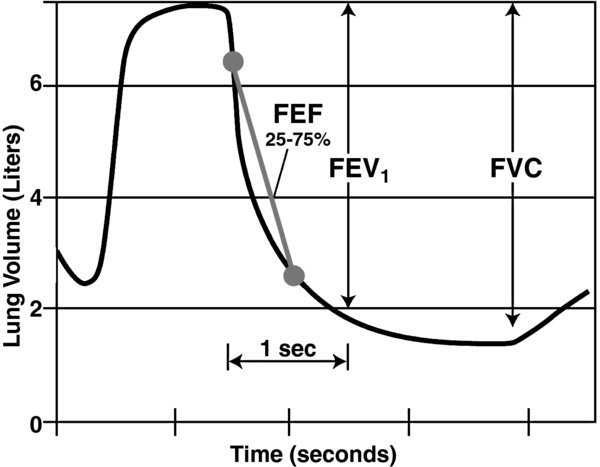
The generated graph is interpreted to determine key flow and volume parameters (Fig. 2).
FIGURE 1. Spirometry involves having the patient take a maximal inspiration after tidal breathing and then forcefully exhaling in what is referred to as a "blast exhalation."
FIGURE 2. Generated graph from spirometry data delineates volumes, volume as a function of time, and flow rates.
- Spirometry parameters. Values are usually expressed as a percentage of normal values. Normal values are defined as mean ±2 SD based on height, age, gender, and ethnicity.
- forced vital capacity (FVC): Maximal volume forcibly exhaled following a maximal inspiration.
- forced expiratory volume in 1 second (FEV1): Single most important measure to determine significant obstructive pathology and to gauge its severity.
- FEV1/FVC is the fraction of the vital capacity (VC) that can be exhaled in 1 second. It is a key metric used to help distinguish between obstructive and restrictive patterns. Decreased values indicate obstruction whereas a normal or higher than normal ratio and reduced lung volumes suggest restrictive physiology.
- forced expiratory flow between 25% and 75% (FEF25-75%) of FVC reflects flow in small airways; it is not effort dependent. The large variability of normal values limits applicability.
- Peak expiratory flow (PEF): Used to monitor responsiveness to bronchodilators; flow must be maintained for at least 10 ms.
- Inspiratory muscles
- Diaphragm: Innervated by the phrenic nerve (C3–C5). Responsible for one-third of the tidal volume when upright and two-third when supine. During contraction, the dome descends (up to 10 cm), elongating the thorax and increasing intra-abdominal pressure.
- External intercostals (intercostal nerves) and accessory muscles (scalene muscles, C4–C8; and sternocleidomastoid, CN XI) both increase the anteroposterior and transverse diameters of the chest.
- Expiratory muscles
- During spontaneous ventilation, expiration at rest is passively driven by the elastic recoil. Inspiratory muscles are relaxed, and no muscles contract.
- During spontaneous ventilation, active expiration (exercise, singing) is generated by the muscles of the abdominal wall and internal intercostals.
Physiology/Pathophysiology
- Spirometry is not a diagnostic test; it reveals respiratory mechanical patterns. These patterns should be reconciled with clinical diagnosis and other diagnostic tests. Once the specific diagnosis is known, spirometry is used to quantify the severity of disease and monitor the progress of therapy.
- Obstructive pulmonary disease (OPD) is characterized by decreased airway flow: Both FEV1 and FEV1/FVC are decreased (FVC is typically normal or increased). The course of disease and response to therapy is best followed by changes in FEV1 (1) [B].
- Emphysema is characterized by enlargement of the airspaces and obstructive chronic bronchitis (OCB) is characterized by excessive mucus production in the bronchi. Severity is assessed per the Global Initiative for Obstructive Lung Disease (GOLD) criteria. Key differential: DLCO is reduced in emphysema but not in OCB.
- Asthma is characterized by airway obstruction, inflammation, hyperresponsiveness to triggers, and the following symptoms: Wheezing, dyspnea, cough, and chest tightness. Diagnostic criteria for asthma: Reduced FEV1/FVC (with no specific ratio); and reversible airflow impairment, defined as an improvement of >12% (or >0.2 L) in FEV1 or FVC. Lack of response does not automatically rule out the diagnosis, as some patients require more aggressive intervention to demonstrate reversibility. Parenchymal gas exchange is preserved to a greater extent than in emphysema, evident by a normal or increased DLCO.
- Restrictive pulmonary disease is characterized by decreases in FVC and either normal or increased FEV1/FVC.
- Interstitial disease (e.g., pulmonary fibrosis) causes decreases in lung volumes, with normal or elevated flow ratios, but decreased DLCO, PaO2, and PaCO2.
- Kyphosis and scoliosis: Depending on severity, may have decreased FVC, FEV1, or both.
- Neuromuscular disorders (amyotrophic lateral sclerosis, multiple sclerosis, Duchenne's muscular dystrophy, myasthenia gravis): Reduced FVC often correlates with severity of disease. Maximal inspiratory and expiratory flows detect muscle weakness.
- Habitus
- Obesity: Decreased chest wall compliance and increased large and small airway resistance result in decreased FVC and FEV1. Also, reduction in volumes and V/Q mismatching.
- Pregnancy: Usually normal spirometry but changes in lung volumes do occur.
- Postoperative pulmonary complications result in prolonged hospital stay and increased health costs (2) [B].
- Reduced lung volumes due to diaphragmatic dysfunction, splinting from pain, respiratory depressant effects of narcotics, and impaired mucociliary clearance are the main mechanisms of pulmonary complications.
- These changes promote postoperative atelectasis, pneumonia, and V/Q mismatches. for example, after thoracic or upper abdominal surgery, FVC and FRC may be reduced up to 60% and 30%, respectively, and can last for 1–2 weeks. Lower abdominal surgery is associated with similar changes but to a lesser degree (3) [B].
- Thoracic surgery: Spirometry is indicated in all patients undergoing lung resection, but for nonlung thoracic surgery it is not always required. (Note: ppo = predicted postoperative)
- Patients with FEV1 >2 L (>80% predicted) tolerate pneumonectomy and those with FEV1 >1.5 L (>60% predicted) can undergo lobectomy (4) [B].
- Increased risk is seen if the FEV1 is <1.5 L; FEV1 <800 mL is generally considered prohibitive for lung resection; a calculated ppoFEV1 <40% suggests an increased risk, <30% high risk
- In borderline patients, DLCO and calculated ppoDLCO (Equation 2) refine the above by assessing parenchymal function; a ppoDLCO <40% is associated with high risk and <20% with unacceptable risk.
- When FEV1 and DLCO are >80% predicted, no further testing is required. for borderline values, integrated cardiopulmonary testing (VO2 max) may be indicated.
- The difficulty of assigning a specific test value prohibitive for lung surgery stems from diversity in patient population (age, sex, gender, race) and variable severity of disease of the remaining lung (e.g., lack of homogeneity of lung disease).
- Perioperative spirometry can aid with risk stratification and planning of risk modification interventions, with the goal of decreasing complications in at-risk patients. Routine testing is NOT indicated in asymptomatic or low-risk patients undergoing nonthoracic, nonabdominal surgery. A thorough history and physical remain the most important tools for perioperative risk assessment. It may be appropriate for:
- Dyspnea or exercise intolerance of unexplained etiology to differentiate between pulmonary and cardiac causes
- Chronic obstructive pulmonary disease (COPD) or asthma where clinical evaluation cannot determine if therapy is optimized
- In general, patients have a high risk for postoperative pulmonary complications when there is an FVC <50% of predicted; FEV1 <50% of predicted, FEV1 <1 L, or postoperative FEV1 <40% of predicted; or significant hypoxemia (paO2 <60 mm Hg) or hypercapnia (paCO2 >45 mm Hg).
- Perioperative risk modification strategies in patients with increased risk of complications (5) [B] include
- Preoperative optimization:
- Asthmatics should have their symptoms controlled prior to surgery, peak flows >80% predicted, or personal best. Anticholinergics and beta-2 agonists given in meter-dosed inhalers are effective. Corticosteroids are useful and are not associated with increased incidence of perioperative infection.
- COPD: Symptom and exacerbation control and optimization
- Use regional anesthesia technique if possible to avoid airway instrumentation.
- Consider a thoracic epidural for thoracic or abdominal surgery to optimize postoperative pain control. This allows for deep breathing, incentive spirometry, and return to early ambulation.
- Use of nonopioid analgesia when applicable; opioids depress the respiratory drive and can increase the paCO2.
- Avoid neuromuscular blockade if possible; when utilized, ensure that the patient is fully reversed.
- Lung protective ventilation strategy includes tidal volumes <8 mL/kg, pressure controlled ventilation, inspiratory pressure <35 cm H2O, PEEP 4–10 cm H2O, and occasional recruitment maneuvers should be considered.
- Careful adjustment of the I:E ratio and ventilatory rate can minimize air trapping (ensures that inspiration starts at FRC).
- Intraoperative delivery of bronchodilators and meticulous pulmonary toilette.
- Bronchospasm can be treated by increasing the depth of inhaled anesthetic, administering bronchodilators (albuterol, ipratropium); meter-dosed inhalers appear to have better medication delivery compared to nebulizers.
- Avoidance of irritating agents such as desflurane during induction and emergence helps prevent bronchospasm.
- Postoperative planning should include adequate monitoring of the environment and the use of noninvasive or invasive ventilatory support.
- Preoperative optimization:
- Predicted postoperative FEV1: ppoFEV1% = FEV1% × (1–fraction of functional lung tissue removed)
- Predicted postoperative DLCO: ppoDLCO% = DLCO% × (1–fraction of functional lung tissue removed). The fraction of functional lung tissue that will be removed can be estimated by CT or ventilation/perfusion scan.