Description
- Functional residual capacity (FRC):
- Is equal to residual volume (RV) plus expiratory reserve volume (ERV)
- Is the volume of air in the lungs at the end of a normal exhalation or tidal volume (TV)
- Participates in alveolar gas exchange, not volume movement. TV serves as the functional component of volume movement gas exchange (“checking account” where most of the activity takes place). Gas (oxygen, volatiles, nitrogen) from the TV readily diffuses into the alveolar units that comprise the FRC (akin to a “volume of distribution”).
- Functions as a “storage” or “reservoir” for O2 in the event of hypoxia or apnea (“overdraft coverage”).
- Corresponds to an intrapleural pressure of 0 cm H2O. The outward rib cage expansion inward lung recoil forces are balanced (“tug-of-war” tie).
- Factors that reduce FRC include: Compression of the thoracic wall, cephalad movement of abdominal contents diaphragm, increased blood volume of the chest/abdomen.
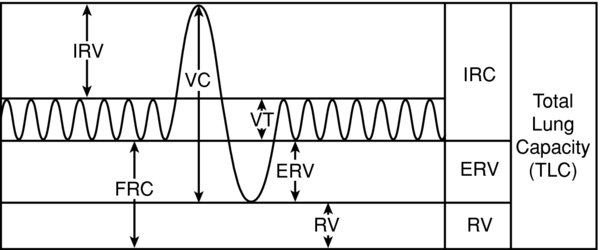
FIGURE 1. Lung volumes.
FRC is the sum of ERV RV. IRV = inspiratory reserve volume, FRC = functional residual capacity, VC = vital capacity, VT = tidal volume, ERV = expiratory reserve volume, RV = residual volume.
- FRC is comprised of the RV ERV. Lung capacities are comprised of lung “volumes.” The lung volumes are measured parameters. FRC is approximately 35 mL/kg (2,500 mL in a healthy 70–kg male).
- The (inward) lung recoil force is referred to as transpulmonary pressure (Ptp). The (outward) chest wall force is referred to as the transmural pressure (Ptm). At end expiration of a normal TV, Ptp Ptm are numerically equal but opposite in sign (positive negative), resulting in an equilibrium.
- FRC during a normal TV does not contribute to the minute ventilation (volume movement). The open alveoli in the FRC, however, will equilibrate with the gas composition in the TV. Thus, FRC functions as a reservoir, or a volume of distribution, in which O2, nitrogen, volatiles, CO2 diffuse to. Assuming normal blood perfusion to the alveolar units, oxygenation, ventilation, gas exchange with the blood take place. During periods of apnea (no new oxygen in the TV), FRC becomes a valuable supply for oxygen. Hence, it provides “overdraft coverage” buffers alveolar gas tensions against abrupt alterations in O2 CO2 during the respiratory cycle.
- ERV during a forced vital capacity (FVC) can participate in volume exchange (open alveoli can contribute to gas exchange).
- RV during a FVC cannot contribute to volume exchange (unable to empty the alveoli from dynamic airway closure/collapse).
- The concept of FRC as a “reservoir” or “savings account” is utilized harnessed when we preoxygenate prior to anesthetic induction. In the event of apnea, the body will have 35 mL/kg of 100% O2 available (e.g., a 70-kg healthy male: 35 mL × 70 kg 2,500 mL × 1.0 FiO2 = 2,500 mL O2; body uses 250 mL O2/minute). This provides additional time before desaturation in the event that ventilation, laryngoscopy, or fiberoptic bronchoscopy are difficult or prolonged.
- Alveoli within the FRC need to be patent/open in order for gas to diffuse into, participate in gas exchange, function as an oxygen reservoir. Obstruction at any point during the breathing cycle or atelectasis may lead to a venous admixture.
- In young healthy individuals, the majority of the alveoli in the FRC (RV + ERV) remain open in the sting position (available for gas exchange reservoir). This is because the closing volume (CV) is less than FRC (CV FRC).
- Closing volume is the volume at which dynamic compression of the airways begins.
Alveoli do not have cartilaginous structures that hold them open but are instead dependent upon proteins (elastin, collagen, trypsin) that surround the alveoli in the parenchyma.
FIGURE 2. The proteins maintain a tight conformational structure that “pull” open the surrounding alveoli (tight coiling effect in parenchyma pulls open the alveoli which are essentially potential spaces).
- When lung volumes reduce (forceful exhalations, FVC, or reductions in FRC), Ptm is increased. When the volume falls below the CV, Ptm exceeds the ability of the parenchymal proteins to keep alveoli open. Atelectasis air trapping or auto-PEEP occurs.
- Alveoli in Zone 4 (lung bases) are most affected during maximal exhalation. They have low resting volumes from gravitational forces on blood (increased Ptm) are vulnerable to compression during active expiration.
- CV is affected by age, smoking, disease states that affect the tight conformational structure of these parenchymal proteins (reduced coiling). Thus, there is reduced alveolar patency increased closing at all lung volumes; gas exchange cannot occur shunting occurs. CV initially infringes upon ERV proceeds into the TV.
- Closing capacity (CC) = CV +RV
- FRC is variable. Factors that affect the Ptp Ptm can increase or reduce FRC. They include the thoracic wall, abdominal wall, diaphragm, blood volume within the lung/abdominal cavities. Meanwhile, CV is not variable but based upon the “health” or “tightness” of the surrounding parenchymal proteins.
- FRC cannot be measured with spirometry; RV cannot contribute to volume exchange during a FVC hence, cannot be directly measured
- Gas dilution techniques: Inhalation of a known concentration volume of an inert tracer gas, such as helium, is performed for 7–10 minutes in a closed circuit (allows for equilibration). The final exhaled helium concentration is diluted in proportion to the unknown volume of air in the patient's chest (RV) can be calculated.
- The nitrogen-washout technique (single-breath multiple-breath techniques): In the single-breath technique, the patient inhales 100% oxygen exhales into a 1-way valve with a nitrogen sampler; creates a curve of nitrogen versus expired volume. At the beginning of exhalation, nitrogen concentration is 0 (dead space contribution that does not participate in alveolar exchange). With continued exhalation, nitrogen concentration starts increasing as alveolar volume is exhaled.
- Bedside ventilator-based FRC measurement techniques are available. There is evidence that FRC measurement (combined with arterial oxygenation respiratory compliance) can help guide assess therapy.
- Lung units: Alveoli, pulmonary capillaries.
- Rib cage: Intercostal, sternocleidomastoid, scalene muscles; xiphoid process, ribs.
- Abdominal contents: Fat, fluid, blood flow, pregnancy.
- Diaphragm: C3, C4, C5 bilateral innervations (affected with interscalene blocks).
Physiology/Pathophysiology
- Factors that reduce lung expansion will reduce FRC result in alveolar collapse/atelectasis air trapping. Atelectasis results in V/Q mismatch (shunting; blood perfuses collapsed alveoli are not oxygenated; gas exchange cannot occur). With increased numbers of alveolar units being shunted, the F(A-a) gradient increases hypoxia results. Furthermore, airway compliance, O2 reservoirs, mixed venous O2 reduce; peak static airway pressures increase.
- Absorption atelectasis: Reductions in FRC also result in air trapping. Absorption of gas behind the closed or intermittently closed airway leads to collapse/atelectasis [hence V/Q mismatch, shunting, increased F(A-a) gradient]. This may be exacerbated by a high FiO2 from near complete uptake of oxygen in the alveoli. Room air partial air mixtures contain nitrogen which is inert poorly dissolved in blood. As a result, nitrogen serves as an alveolar “filler,” reducing complete collapse (like foam packaging in a box; if compressed, less likely to be completely collapsed).
- The outward chest wall force (Ptp) inward lung recoil (Ptm) determine the FRC (intrinsic factors). However, they are altered by factors that affect the thoracic wall, abdominal wall, diaphragm, blood volume. These factors are variable, extrinsic, often affected by anesthesia surgery. FRC is still the volume at the end of a normal TV, Ptp + Ptm continues to equal 0 mm Hg; however, lung volume at FRC will be re-established
- Thoracic wall: Reduction in diameter cross-sectional area are seen with restrictive lung diseases affecting the chest wall—scoliosis, muscle dystrophies (ALS, paraplegia, etc.), obesity (fat on the chest).
- Age affects chest wall compliance. Babies have very compliant thorax lung (allows for easy passage through birthing canal); FRC is small roughly equal to RV. They also have a low O2 reservoir increased O2 consumption ( 6 mL O2/kg/minute, double that of older children adults) that results in rapid desaturation during apnea (1).
- Elderly have ossified thoraxes that become stiffer increase outward forces FRC (3% per decade). CV, however, increases at a greater rate, leading to airway closure, atelectasis, V/Q shunting.
- Inspiratory muscle tone affects the intercostals, sternocleidomastoid, scalene muscles during GA /or NMBD (reduces thoracic outward force, FRC) (2).
- Barrel chest, seen with emphysema, increases chest wall diameter, Ptm, TLC, hence FRC. It is a compensatory mechanism to reduce atelectasis in the face of an increased CV from damaged parenchymal proteins that are unable to maintain patent alveoli.
- Abdominal wall: Obesity, pregnancy, ascites, abdominal thoracic surgery, abdominal retractors packing, pneumoperitoneum, supine Trendelenburg position allow for cephalad movement of abdominal contents. This results in external compression or “invasion” into the lung cavity an increase in Ptp (reduces FRC).
- Diaphragm: Intrinsic muscle tone is reduced with NMBD anesthesia (IV volatile), reducing diaphragmatic excursion lung expansion (reduced FRC). Bilateral diaphragm paralysis (cervical quadriplegia, surgical injury, radiation) produces similar effects on FRC.
- Blood volume within lung/abdominal cavities as seen during surgery, congestive heart failure (CHF), acute respiratory distress syndrome (ARDS), fluid overload states reduce lung expansion hence FRC.
- CV, unlike FRC, has limited variability.
- An abnormal increase in FRC can result from obstructive airway diseases (asthma, reactive airway disease, emphysema, chronic bronchitis) due to an increase in RV, at the expense of ERV. The increased RV cannot contribute to the exhaled volume; gas exchange can occur in alveoli that open with inhalation/increased lung volumes, despite closing with exhalation (decreased lung volumes).
- Intraoperative shunt is greater in patients with pre-existing low PaO2, but the percentage increase is larger in healthy subjects
- Reductions in FRC may be significant intraoperatively perioperatively. Intraoperative shunt is greater in patients with pre-existing low PaO2, but the overall percentage is larger in healthy subjects.
- Supine position (0.8 L) induction of general anesthesia (0.5 L) are not inconsequential (1.3 L reduction; reduces lung volumes from 3.5 to 2.2 L, brings closer to, may overlap CV) (1)
- Although positive pressure ventilation reduces the effects of the diaphragm abdominal wall on FRC, it does not negate it entirely (3).
- Implementing large TV (10–12 mL/kg) PEEP may increase FRC gas exchange. Opens atelectatic alveoli during inspiration (allows O2 CO2 to be exchanged with blood); consider pressure control ventilation or an increased inspiratory pause time (increases time that alveoli are open during inspiration).
- PEEP preferentially affects upper lung zones; airway closure reduced FRC predominantly affect dependent lung zones. Higher PEEPs may improve airway patency in dependent lung regions but can reduce cardiac output pulmonary perfusion (V/Q mismatching, dead space)
- 30° head-up tilt (not back up) allows gravity to bring the abdominal contents downward, increase FRC, reduce atelectatic units.
- High FiO2 that is often used perioperatively can result in absorption atelectasis in poorly ventilated or “closed” alveoli.
- After intubation, early recruitment maneuvers (up to 40 cm H2O for 10 seconds) may reduce atelectasis.
- Coughing during intubation, intraoperatively, or extubation reduces FRC. Avoid with adequate induction NMB medication dosage time for onset. Consider recruitment maneuvers prior to extubation.
- Postoperative reductions in FRC can cause hypoxemia, atelectasis, pneumonia; different etiology than intraoperative decreases. Results from absorption atelectasis (high O2 behind closed airways), incisional pain, reflex dysfunction of the diaphragm (local irritation, bowel distension, pneumoperitoneum) after abdominal thoracic surgeries
- Active lung expansion: Deep breathing exercise coughing, incentive spirometry, CPAP. Intensity frequency of sessions are more important than form of therapy.
- Postoperative analgesia: Epidural analgesia may allow for deep breathing diaphragmatic excursion.
- FRC = RV + ERV; FRC = TLC – IRV – TV; where FRC is functional residual capacity, RV is residual volume, ERV is expiratory reserve volume, TLC is total lung capacity, IRV is inspiratory reserve volume, TV is tidal volume.
- CC = CV + RV; where CC is closing capacity, CV is closing volume, RV is residual volume.