Blood is a “liquid organ” with extraordinary and unique functions. Blood carries oxygen to cells, carries waste away from cells, contains disease-fighting cells, and helps in regulation of body pH and temperature. Fifty-five percent of blood is composed of plasma (fluid); the remaining cellular portion (45%) is made up of solids (RBCs, WBCs, and platelets). Blood components are briefly described in the following section. In Table 11-6, volumes, actions and indications, infusion guidance, and special considerations for the more commonly transfused blood components are summarized.
| Blood Component | Volume | Actions and Indications | Infusion Guide | Special Considerations |
|---|---|---|---|---|
| Red blood cells (RBCs) | 225-350 mL | Symptomatic anemia Hemoglobin level of 7-8 g/dL in stable, hospitalized patients Hemoglobin 8 g/dL or less in the presence of clinical symptoms for patients with cardiovascular disease | Standard blood filter (150-260 microns) Y administration set, primed with 0.9% sodium chloride Transfuse each unit in 4 hours or less | Crossmatch required ABO and Rh compatible Each unit raises the hemoglobin 1 g and hematocrit 3% Smaller aliquots of RBC volume can be made for use with neonatal, pediatric, or adult patients with special needs |
| Granulocytes | 200-300 mL | Transfusion of granulocytes controversial Treatment of neutropenic patients with documented infections unresponsive to antimicrobial therapy | Standard blood filter Y administration set, primed with 0.9% sodium chloride No leukocyte-reduction filter or depth-type microaggregate filters Administer slowly over 2-4 hours | Crossmatch required ABO and Rh compatible Monitor closely for reactions May be HLA matched Note: High frequency of febrile nonhemolytic reactions Premedication used (e.g., diphenhydramine, steroids, nonaspirin antipyretics) |
| Platelets, random donor | 40-70 mL/unit Usual dose: 4-10 units | Bleeding due to thrombocytopenia or platelet function abnormality including antiplatelet drugs Prevention of bleeding from marrow hypoplasia | Standard blood filter Y administration set, primed with 0.9% sodium chloride Administer as rapidly as patient can tolerate; generally transfused over less than 4 hours; usually 15-30 minutes Leukocyte reduction if indicated | ABO/R compatibility preferred but not required Crossmatch not required ABO/Rh preferred (the amount of RBCs and platelets harvested with the platelets is generally minimal but occasionally is sufficient to elicit an antigen-antibody response) May be HLA matched Prophylactic medication with antihistamines, antipyretics may be needed |
| Fresh frozen plasma (FFP) | Prepared from whole blood 200-250 mL | Active bleeding with multiple coagulation factor deficiencies Warfarin reversal in the presence of bleeding or in the need for an invasive procedure (e.g., surgery) Disseminated intravascular coagulation (DIC) Massive transfusions in trauma | Standard blood filter First 15 minutes: 2-5 mL per hour, then as rapidly as tolerated (up to 300 mL per hour); usually 30-60 minutes | Crossmatch not required ABO compatible Does not provide platelets 1 unit (200 mL) raises the level of clotting factor 2%-3% Requires 20 minutes of thawing time by transfusion service; infuse immediately |
| Cryoprecipitated antihemophilic factor (AHF) | Each unit contains factor VIII, von Willebrand factor (vWF), factor XIII, fibrinogen 15 mL plasma (5-10 mL/unit) Usual order is for 6-10 units | Main use today as a source of fibrinogen in acquired coagulopathies: DIC, massive hemorrhage Alternative to factor VIII | Standard blood filter Small priming volume tubing set to decrease loss of product Administer over less than hours; usually 15-30 minute | Crossmatch and ABO compatibility not required Infuse as soon as possible after thawing |
| Albumin (5% = 12.5 g/250 mL; 25% = 12.5 g/50 mL) | 5% solution is in concentration of 250 or 500 mL; 25% solution is in concentration of 50-100 mL | Plasma volume expander For hypovolemic shock Supports blood pressure during hypotensive episodes; induces diuresis in fluid overload | May be administered as rapidly as tolerated for reduced blood volume Normal rates: 2-4 mL/min for 5% solution;1 mL/min for 25% solution Supplied in glass bottles with tubing for administration | 25% albumin is hypertonic and is five times more concentrated than 5% solutions. Give with extreme caution; can cause circulatory overload No type and crossmatching necessary Store at room temperature |
| Plasma Protein Fraction (PPF) (5% solution) | Glass: 250-mL bottle with tubing (83% albumin, 17% globulin) | Same as for albumin Osmotically equal to plasma | Equivalent to 5% albumin | Has fewer purification steps than albumin No type and crossmatching necessary Has high sodium content |
Source: AABB, 2018; Jorgenson, 2020.
Whole Blood
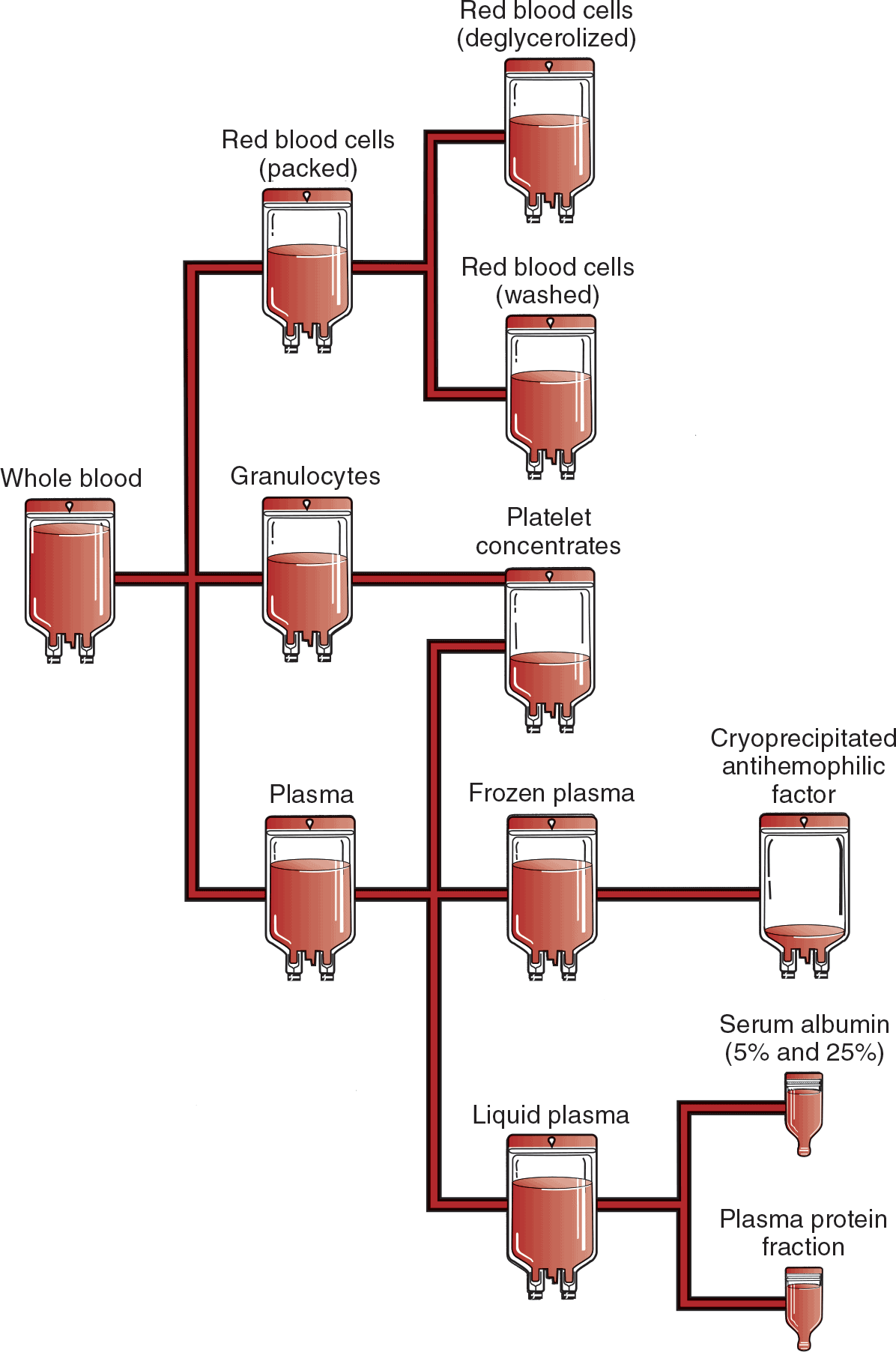
Whole blood is composed of RBCs, plasma, WBCs, and platelets. The volume of each unit is approximately 500 mL and consists of 200 mL of RBCs and 300 mL of plasma, with a minimum of 33% Hct. Few conditions require transfusion of whole blood (Shehata & Mo, 2020). Most whole blood units are used to prepare separate RBC and plasma components to meet specific clinical needs. A whole blood unit can be centrifuged and separated into three components: RBCs, plasma, and platelet concentrate. By transfusing the patient with the specific component needed rather than with whole blood, the patient is not exposed to unnecessary portions of the blood product, and valuable blood resources are conserved (Fig. 11-4). Whole blood requires type and crossmatching and must be ABO compatible.
Red Blood Cells
RBC units are prepared by removing 200 to 250 mL of plasma from a whole blood unit. The remaining packed red blood cells (PRBCs) concentrate has a volume of approximately 225 to 350 mL. Each unit contains the same RBC mass as whole blood, as well as 20% to 30% of the original plasma, leukocytes, and some platelets. The advantages of RBCs over whole blood are decreased plasma volume and decreased risk of circulatory overload. Another advantage is that because most of the plasma has been removed, less citrate, potassium, ammonia, and other metabolic by-products are transfused.
Uses
RBCs are used to improve the oxygen-carrying capacity in patients with symptomatic anemia. The administration of RBCs should be considered only if improvement of the RBC count cannot be achieved through nutrition, drug therapy, or treatment of the underlying disease. As stated earlier, transfusions should be restricted to an Hgb level of 7 to 8 g/dL in stable, hospitalized patients and to 8 g/dL or less in the presence of clinical symptoms for patients with cardiovascular disease (Carson et al., 2012; Frank & Guinn, 2020). RBCs should not be used to treat anemia that can be corrected with hematinic medications (e.g., iron, vitamin B12, folic acid, erythropoietin). Criteria for transfusion should be based on multiple variables, including Hgb and Hct levels, patient symptoms, amount and time frame of blood loss, and surgical procedures.
Leukocyte-Reduced Red Blood Cells
Leukocyte reduction is achieved either by using filtration before transfusion or by using a special filter at the bedside during transfusion. Leukocyte-reduced RBCs require ABO compatibility (Fig. 11-5). Leukocyte-reduced components are indicated as follows:
- To reduce the frequency of recurrent febrile nonhemolytic transfusion reactions
- To prevent transmission of cytomegalovirus (CMV)
- To reduce the incidence of HLA alloimmunization
Safety Concern: On rare occasions, patients may develop severe hypotension when leukocyte reduction is performed at the bedside. This happens more often in patients who take angiotensin-converting enzyme (ACE) inhibitors (Jorgenson, 2020). In general, leukocyte reduction at the time of collection is preferred over filtration at the bedside.
Washed Red Blood Cells
RBCs can be “washed” with sterile normal saline to remove unwanted plasma proteins, including antibodies and glycerol from previously frozen units. There are limited clinical indications for washed RBCs. They include large-volume infusions to potassium-sensitive patients, for patients who have a history of plasma-related transfusion reactions, and to mitigate TRALI (Acker & Razatos, 2020).
Irradiated Blood Products
Cellular blood components can be irradiated for patient populations at risk for transfusion-associated GVHD (Hod & Francis, 2020). These patients include fetal and neonatal recipients of intrauterine transfusions, immunocompromised patients, recipients of cellular components known to be from a blood relative, and those who have undergone bone marrow transplantation.
 NURSING FAST FACT!
NURSING FAST FACT!A label marked “irradiated” must be placed on the bag containing the product. Whole blood, RBCs, platelets, or granulocytes can be irradiated. Patients with sickle cell and neonates may require blood tested as negative for hemoglobin S (AABB, 2018). |
The primary indication for granulocyte transfusion is in the treatment of prolonged severe neutropenia with intensive chemotherapy for hematological cancers (Shehata & Mo, 2020). Granulocyte concentrations are prepared by apheresis from a single donor of whole blood. Each unit contains granulocytes and variable amounts of lymphocytes, platelets, and RBCs suspended in 200 to 300 mL of plasma.
Granulocyte infusions should be administered as soon after collection as possible because of the well-documented possibility of deterioration of granulocyte function during short-term storage. The role of granulocyte transfusion is not well defined and clinical judgment is imperative in decision-making (Shehata & Mo, 2020).
Platelets
There are approximately 2.2 million doses of platelets transfused each year in the United States, primarily for prophylactic indications to reduce bleeding risk in patients with thrombocytopenia after cancer chemotherapy or hematopoietic progenitor cell transplantation (Kaufman et al., 2015). Platelets must be stored at room temperature and therefore have a limited shelf life of only 5 days due to the risk of bacterial growth.
Platelets live up to 12 days in the blood, do not have nuclei, and are unable to reproduce. They contain no hemoglobin. Normal platelet counts are 150,000 to 300,000/µL. Platelets can be supplied either as random-donor concentrates or from single-donor apheresis. Random-donor concentrates are prepared from individual units of whole blood by centrifuging the unit to separate the platelets. Single-donor platelet apheresis products are collected from a single donor. During pheresis, the platelets are harvested, and all unneeded portions of the blood are returned back to the donor. A single pheresis unit is equivalent to 6 to 8 units of random-donor platelets (Fig. 11-6).
Use of a single-donor unit has the obvious advantage of exposing the recipient to fewer donors and is ideal for treating patients who have developed HLA antibodies from previous transfusions and have become refractory (unresponsive) to random-donor platelets. HLA typing may be indicated when patients become refractory to platelets after multiple transfusions.
 NURSING FAST FACT!
NURSING FAST FACT!One unit of platelets would be expected to increase the platelet count of a 70-kg adult by 5000 to 10,000/µL and to increase the count of an 18-kg child by 20,000/µL (AABB, 2013). |
The effectiveness of platelet transfusions may be altered if fever, infection, or active bleeding is present. To determine the effectiveness of a transfusion, platelet counts may be checked 1 hour and 24 hours after transfusion. Poor platelet count recovery may also indicate that the patient is refractory to random-donor platelets.
Plasma Derivatives
Plasma and Fresh Frozen Plasma
Plasma is the liquid portion of the blood that is prepared by separating the plasma from the unit of whole blood. It contains albumin, globulins, antibodies, and clotting factors. Plasma does not contain any cellular portion of blood. Indications for plasma transfusions include patients with active bleeding, at risk for bleeding due to coagulation factor deficiencies, and anticoagulant (e.g., warfarin) reversal in the presence of bleeding or in the need for an invasive procedure.
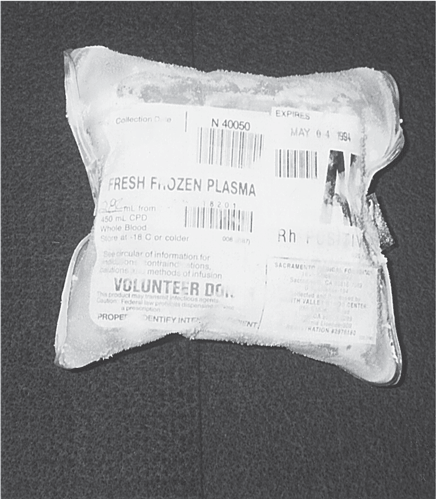
Fresh frozen plasma (FFP) is prepared from whole blood by separating and freezing the plasma within 8 hours of collection. This preserves the levels of coagulation factors V and VIII (Shehata & Mo, 2020). The volume of a typical unit is 200 to 250 mL (Fig. 11-7). The thawing process takes up to 30 minutes; after thawing, the FFP should be transfused immediately or refrigerated and used within 24 hours.
Cryoprecipitated Antihemophilic Factor
Cryoprecipitated antihemophilic factor (AHF) is a plasma derivative. Indications for its use are limited because there are recombinant factor replacement products available today for the treatment of hemophilia. It is used when no factor-specific concentrate is available to treat conditions such as correcting low fibrinogen levels, improving poorly functioning fibrinogen, treating von Willebrand disease, and correcting low factor VIII, XIII, or fibrinonectin levels (AABB, 2018).
Recombinant Factor Replacement Products
Today, most of the factor replacement products used in managing hemophilia are derived from recombinant DNA rather than processed from donated plasma.
Administration
- Usually administered by slow IV bolus infusion in the acute, outpatient, or home-care environment. Infusion time varies by product, so it is important to follow the manufacturer's directions.
- Packaged in kits that include a vial of factor (powder), a diluent, and a mixing device; most with a built-in filter. Sometimes, a steel needle IV device is included in the kit.
- Generally supplied by the blood center. Stored in refrigerator or at room temperature (as directed by the manufacturer); shelf life up to 2 years.
- Dosage varies and is based on units per kilogram.
Factor VIII
Hemophilia A is a deficiency of coagulation factor VIII that occurs in about 1:10,000 males (Hitch, 2013). Factor VIII replacement either is used routinely for patients with severe hemophilia or may be used situationally in moderate to mild cases, such as patients undergoing prophylactic therapy with planned surgery or with a traumatic event.
Hemophilia B is a deficiency of coagulation factor IX that occurs in about 1:50,000 males (Hitch, 2013).
Safety Concern: Patients can develop antibodies to replacement factor, making it more difficult to control bleeding. This may require higher doses or a bypassing agent that allows clotting to occur without factor VIII or IX (Hitch, 2013).
Albumin and Plasma Protein Fraction
Albumin is a natural plasma protein commercially extracted from plasma. It supplies 80% of the osmotic activity of plasma and is the principal product of fractionation (dividing plasma into its component parts). It is administered as plasma protein fraction (PPF) or purified albumin. Both products (albumin and PPF) are derived from donor plasma, prepared by the cold alcohol fractionation process, and then subsequently heated. Because of the extended heating process, neither product transmits viral diseases, and neither product contains clotting factors. Normal serum albumin is composed of 96% albumin and 4% globulin and other proteins. It is available as a 5% (isotonic) or 25% (hypertonic) solution. PPF is available only in a 5% solution.
NOTE: Additional information on albumin as a plasma expander is provided in Chapter 4.
PPF and 5% albumin are isotonic solutions and therefore are osmotically equivalent to an equal volume of plasma. They cause a plasma volume increase, are used interchangeably, and share the same clinical uses. Both are used primarily to increase plasma volume after sudden loss of intravascular volume, as seen in patients with hypovolemic shock from trauma or surgery. Their use may also be indicated in individual cases to support blood pressure during hypotensive episodes or to induce diuresis in those with fluid overload by assisting in fluid mobilization. The plasma derivatives lack clotting factors and other plasma proteins and therefore should not be considered plasma substitutes. Neither component (albumin or PPF) will correct nutritional deficits or chronic hypoalbuminemia.
Twenty-five percent albumin is hypertonic and is five times more concentrated than 5% albumin. This hyperosmotic product is used to draw fluids out of tissues and body cavities into intravascular spaces. This solution must be given with caution. Principal uses for 25% albumin include plasma volume expansion, hypovolemic shock, burns, and prevention or treatment of cerebral edema.
 NURSING FAST FACT!
NURSING FAST FACT!Albumin 25% must not be used in dehydrated patients without supplemental fluids or in those at risk for circulatory overload. |
Administration
Albumin and PPF are supplied in glass bottles. Manufacturers recommend that the solution be used within 4 hours of opening. Depending on the manufacturer, the solutions are sometimes supplied with an infusion set. If no administration set is provided, a standard administration set without a filter is used. Blood transfusion sets and filters are not required for infusion of albumin.