Historically, control of the IV rate was regulated primarily with a roller clamp, which the nurse adjusted manually on the administration set. Although roller clamp rate control is still useful in some situations, mechanical infusion devices and electronic infusion pumps are more commonly used to maintain accurate infusion rates. The term flow-control device refers to any instrument used to regulate infusion flow rate, including:
- Manual devices (e.g., gravity infusion/roller clamp)
- Nonelectronic flow control (e.g., elastomeric pumps, spring-based pumps)
- Electronic infusion pumps (Gorski et al., 2021).
Gravity Driven Infusions Using Manual Flow Control
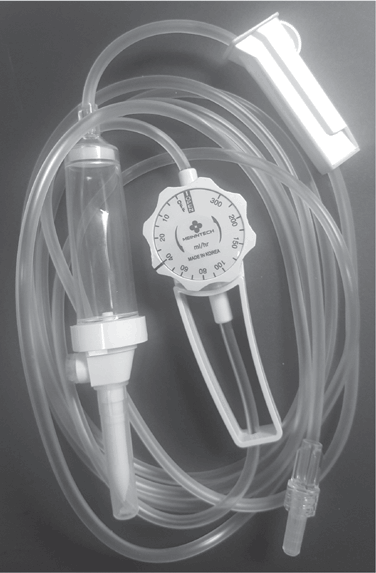
Manual flow control includes use of the roller clamp of the IV tubing or, alternatively, use of a “manual flow regulator” in lieu of the roller clamp for a gravity infusion. The manual flow regulator allows the nurse or patient to set the flow rate in milliliters per hour. Advantages may include easier regulation, more consistent flow, less drifting of flow compared to using the roller clamp, and less risk of accidental free flow. However, the accuracy is about the same as with the roller clamp (±10%). Manual flow regulators are set to deliver specified volumes of fluid per hour. They are available as dials, with clocklike faces, or as barrel-shaped devices with cylindrical controls (Fig. 5-34). Flow markings on the dials help to approximate the drops per minute based on the set drop factor. Because these devices are gravity based, it is important to recognize that a number of factors affect the accuracy of the flow, such as patient position, height of the infusion container, and decreased volume in the solution container. Flow rate should be verified by counting the drops. These devices are useful in certain situations in which some variation in flow rate is not critical. Examples include home infusions of subcutaneous fluids or certain antibiotics.
 NURSING FAST FACT!
NURSING FAST FACT!When using a manual flow regulator, always verify the flow rate by counting the drops. |
Gravity Infusions Using an Electronic Drip Monitor
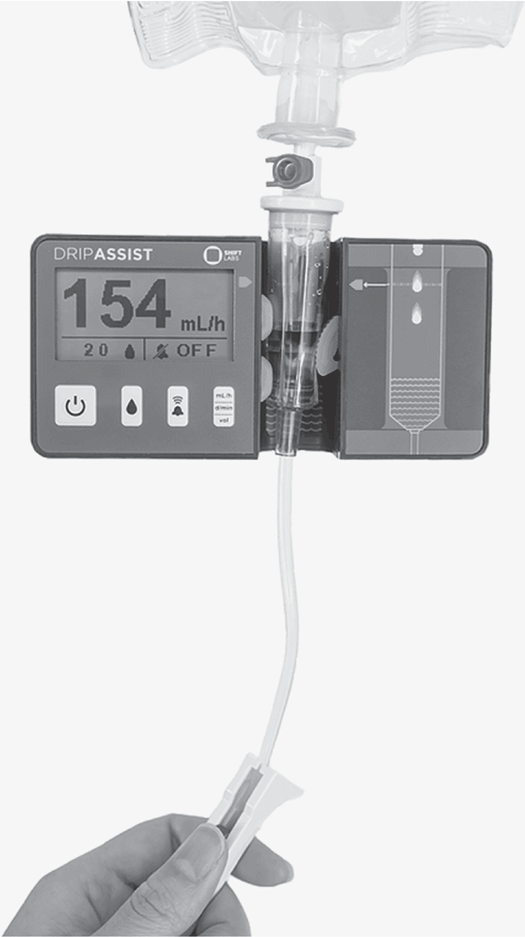
This device is placed around the drip chamber to monitor the flow rate. The nurse selects the administration set drop/mL factor (as listed on the administration set container) and adjusts the roller clamp to the right drop rate, and the device monitors the rate. An alarm sounds if the drip rate changes (Fig. 5-35).
Mechanical Infusion Devices (Nonelectric)
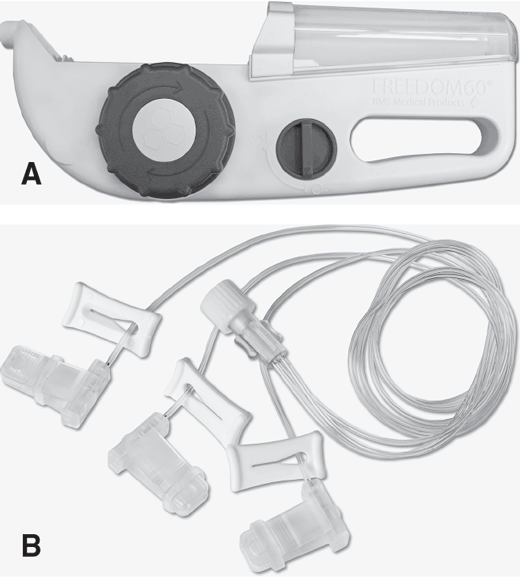
Mechanical infusion devices use no electricity and utilize various methods to infuse therapies via positive pressure. Certain devices, considered variable pressure, utilize a stretched elastomer or compressed spring to create the force required to infuse, with the decreasing force leading to decreasing flow rates throughout the infusion. One device uses a constant pressure mechanism that maintains flow rate until acted upon through feedback. Based on the increasing pressure in the area that the infusion is entering the patient, there is a decrease in flow rate and reduction in site reactions (Fig. 5-36A). Tubing diameter and length have a determining influence on the device's initial flow rate (Fig. 5-36B). Such constant pressure devices can be used to administer both subcutaneously or intravenously. These types of flow-control devices are more widely used in non-acute care settings.
The elastomeric pump system is a portable, single-use device with an elastomeric reservoir, or balloon. The balloon, which is made of a soft rubberized material capable of being inflated to a predetermined volume, is encapsulated inside an outer case that may be a soft- or hard-shelled transparent container. The pump includes preattached tubing, an inline filter, and built-in clamp. When the reservoir is filled, the balloon exerts positive pressure to administer the medication with an integrated tubing that controls the flow rate (Fig. 5-37). Used most often in home or outpatient settings, the elastomeric pump is used to deliver a variety of infusion therapies including IV antibiotics, chemotherapy, and analgesics.

Advantages
- Portability
- Simplicity
- Patient education is simplified; many patients learn in one to two teaching sessions
Disadvantages
- Cold infusates slow infusion rate; infusion solutions should be at room temperature.
- Viscosity of fluid will have an inverse effect on flow rate.
- Atmospheric pressure can affect flow accuracy.
- More costly.
- Limited number of medications compatible with system.
 NURSING FAST FACT!
NURSING FAST FACT!Allow elastomeric infusion pumps to warm after storage and before infusion to improve accuracy in the flow rate. |
Electronic Infusion Pumps
Electronic infusion pumps are powered by electricity or battery and are programmed to provide accurate regulation of the IV flow rate. They can be classified in a number of ways such as by how they work, portability (i.e., ambulatory), type of container (e.g., syringe pumps), and infusion modes (e.g., patient-controlled analgesia [PCA]).
Electronic infusion pumps deliver infusions using positive pressure. The normal pumping pressure is slightly lower than the occlusion pressure. Some pumps have a preset or fixed occlusion pressure, whereas others allow the nurse to change the occlusion pressure. It is important to recognize that occlusion pressures are a safety feature, and nurses should be cautious when changing the pressure to avoid setting off alarms. Sometimes it may be appropriate to increase the pressure, such as with high-volume high-pressure infusions, arterial infusions, and those delivered in a hyperbaric chamber.
Electronic infusion pumps are used in IV infusions as well as with subcutaneous, arterial, and epidural infusions. These devices provide an accurate flow rate and have alarms that signal problems with the infusion. However, regular assessment, responsibility, and accountability for safe infusion lie with the nurse. To use these devices effectively, the nurse should know (1) indications for their use, (2) their mechanical operation, (3) how to troubleshoot, (4) their psi rating, and (5) safe usage guidelines.
Volumetric Pumps
Volumetric pumps work by having a cassette or reservoir of a measured chamber volume placed inside the pump. They infuse a known volume of fluid with every cycle. The mechanism of action involves filling and ejection of the chamber, which causes a pulsatile flow that can cause greater error at lower infusion rates (Kan & Levine, 2021). Volumetric pumps require pump-specific administration sets.
 NURSING FAST FACT!
NURSING FAST FACT!To ensure safe, efficient operation, review the literature that accompanies the pump to become familiar with its operation. Observe all precautions. |
INS Standard Administration sets with anti-free flow mechanisms are used with electronic infusion pumps (Gorski et al., 2021, p. S69).
Peristaltic Pumps
Peristaltic refers to the controlling mechanisms: A peristaltic device moves fluid by intermittently squeezing the IV tubing. The device may be rotary or linear. In a rotary peristaltic pump, a rotating disc or series of rollers compresses the tubing along a curved or semicircular chamber, propelling the fluid when pressure is released. In a linear device, small finger-like projections intermittently press the IV tubing. Rotary peristaltic pumps are used primarily for infusion of enteral feedings (Kan & Levine, 2021).
Ambulatory Electronic Infusion Pumps
Ambulatory pumps are lightweight, compact infusion pumps. They are used primarily for patients requiring home infusion therapy, allowing the patient mobility and freedom to resume a normal life. Ambulatory electronic infusion pumps are capable of delivering most infusion therapies, including continuous infusions (e.g., chemotherapy and inotropes), intermittent antibiotic therapy, analgesic infusions with PCA, and continuous infusions with tapering functions (e.g., PN) (Fig. 5-38). For intermittent antimicrobial infusions, a “keep vein open (KVO) rate” is programmed to maintain flow between the drug administrations. Features include programmable memory, lockout functions for safety, and alarms. “Smart pumps” that include drug libraries are available. Ambulatory pumps function on a battery system that requires recharging or replacement of disposable batteries. The pump, along with the infusion container, is placed in a pouch or backpack, providing the patient with full mobility during the infusion.

Electronic Syringe Pumps
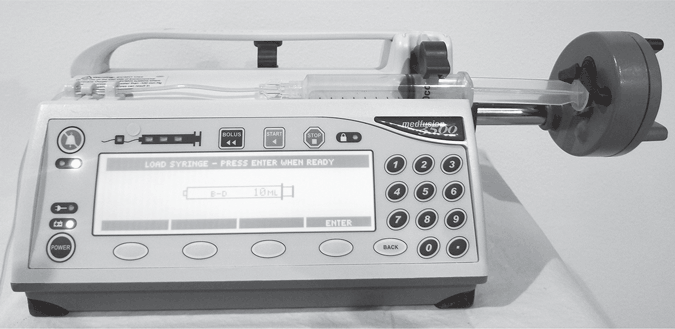
Electronic syringe pumps use a traditional syringe as the solution container, which is filled by the pharmacy with prescribed medication and positioned in a special pump designed to hold it. Syringe pumps are valuable for critical infusions of small doses of high-potency drugs. They are precisely accurate delivery systems that can be used to administer very small volumes. Some models have program modes capable of administration in milligrams per kilogram per minute, micrograms per minute, and milliliters per hour.
These pumps are used most frequently for delivery of antibiotics and small-volume parenteral therapy. Syringe pump technology is available for PCA infusion devices. Syringe pumps are used frequently in the areas of anesthesia, oncology, pediatrics, neonatal intensive care units, home care, and obstetrics.
The volume of the syringe pump is limited to the size of the syringe; a 60-mL syringe is generally the largest a syringe pump can handle. However, the syringe can be as small as 5 mL. The tubing usually is a single, uninterrupted length of kink-resistant tubing with a notable lack of Y injection ports (Fig. 5-39).
Patient-Controlled Analgesia Pumps
PCA pumps are used for pain management across all care settings, including acute care, long-term care, hospice, and home. PCA pumps can be used to deliver medication via the IV, subcutaneous, and intraspinal routes. These pumps are distinct from other electronic infusion pumps in that a remote bolus control allows the patient to deliver a bolus of medication at set intervals by pressing a button on a cord. PCA pumps are available in ambulatory or pole-mounted models and, as mentioned earlier, PCA technology is available with syringe pumps as well.
The PCA pump can be programmed to deliver a continuous infusion, a demand infusion, or both (see Chapter 10) All three afford pain control with varying degrees of patient interaction. Some pumps offer oxygen saturation monitoring, which sets off alarms and potentially stops the infusion in the event of hypoxemia.
- The continuous infusion is designed for patients who need maximum pain relief without the need for on-demand dosing. May be used for intraspinal infusions and for patients unable to use the demand function.
- The demand-mode infusion dose is delivered by intermittent infusion when a button attached to the pump is pushed. The demand dose can be used alone or with a continuous basal type of infusion.
- The basal mode refers to the continuous delivery of pain medicine in conjunction with the demand mode. Should the patient require additional pain medication, for example, as associated with increased activity or a painful procedure, the demand dose is delivered in conjunction with the basal rate. This mode is often used in palliative care and hospice settings for patients with chronic pain.
 NURSING FAST FACT!
NURSING FAST FACT!PCA pumps are designed with a special key or locking device for security of the medications. |
Multichannel Pumps
Multichannel pumps can deliver several medications and fluids simultaneously at multiple rates from bags, bottles, or syringes. They are used most often in critical care settings. Multichannel pumps (usually with two to four channels). Whether or not they are in use, each channel requires manifold-type sets to set up all channels, whether or not they are in use; each channel must be programmed independently (Fig. 5-40).
Smart Pumps
Smart pumps refer to electronic infusion pumps with a dose error reduction software (DERS). The DERS is the computer software, a drug library, within the smart pump designed to prevent infusion programming errors and warn users of potential over- or under-delivery of a medication or fluid. Programmable infusion pumps with DERS systems help to avert potentially harmful errors by “remembering” the large number of “rules” (hospital-defined dosing limits and other clinical advisories) entered into the drug library, and applying those “rules” during pump programming to warn clinicians about potentially unsafe drug therapy (Institute for Safe Medication Practices ([ISMP], 2020, p. 9). The ISMP recommends their use for all medication infusions. Features of smart pumps are listed in Table 5-2.
| Feature | Description |
|---|---|
| Drug library | A comprehensive list of medications and fluids that can be delivered. Includes drug names, concentrations, dosing units, hard and soft dose limits, maximum rates, volumes. |
| Soft limit | An alert that can be overridden by the user; a soft limit will alert the user that a dose is unusually low or high, but the user can still proceed with the infusion. |
| Hard limit | An alert that cannot be overridden by the user; a hard dose limit cannot use a dose that is higher or lower than what is programmed into the pump. Indicates that the dose is out of the organization's safe range |
| Clinical advisory | An alert that prompts the nurse to consider actions that are relevant to the medication, such as the use of a certain type of tubing or the use of a filter |
| Data logs | Data are continuously collected, including alerts, medications, programmed doses, actions taken Useful in quality improvement efforts |
| Continuous display | Drug name and dosage displayed on screen at all times |
| Wireless technology | Drug libraries and software updates can be sent wirelessly to all pumps in an organization. |
Smart pumps are not without limitations. The accuracy of information entered into the smart pump (e.g., patient weight) is dependent on correct data. If the nurse bypasses the drug library and manually enters the infusion rate and volume, the DERS will not be able to identify potential errors. Smart pumps do not detect errors related to wrong patient selection, wrong drug library selection, or bypassing the drug library (Phelps, 2017). Smart pump technology is incorporated into the pump shown in Figure 5-40. Important to implementing smart pumps is the creation of a comprehensive drug library with pharmacist involvement, standardizing drugs across specialties, and education (Shah & Jani, 2020).
The ability of medical devices, such as pumps, to exchange information with other devices or systems is called medical device interoperability. Smart pumps have the capacity to connect and share data with a centralized server through wired, wireless, or other networking capabilities (Kan & Levine, 2021). Errors are reduced when smart pumps and the electronic prescribing system communicate through systems including computerized physician order entry (ISMP, 2021).
INS Standard Consider the use of electronic infusion pumps with DERS for IV administration of medication and solutions throughout the acute care setting as they are associated with reduced risk for infusion-related medication errors, including error interceptions (e.g., wrong rate) and reduced adverse drug events (Gorski et al., 2021, p. S69).
 NURSING FAST FACT!
NURSING FAST FACT!It is important to refer to the manufacturer's directions for setup and troubleshooting guidelines of each electronic infusion pump. |
Electronic Infusion Pumps: Pump Programming
Electronic infusion pumps are programmed based on the parameters of the specific infusion therapy. Parameters include:
- Rate: Amount of time over which a specific volume of fluid is delivered. Infusion pumps deliver in increments of milliliters per hour. The most common rate parameters for regular infusion pumps are 1 to 999 mL/hr. Some pumps are capable of setting rates that offer parameters of 0.1 mL in increments of 0.1 to 99.9 mL, then in 1-mL increments up to 999 mL. Many newer pumps are capable of setting rates that satisfy both regular infusion and microinfusion needs.
- Volume infused: Measurement that tells how much of a given solution has been infused. This measurement is used to monitor the amount of fluid infused in a shift. It also can be used in home health to monitor the infusion periodically during the day or over several days.
- The “counter” is generally returned to 0 at the beginning of each shift.
- Volume to be infused: Usually the amount of solution hanging in the solution container. A pump is designed to sound an alarm when the volume to be infused is reached.
- Tapering or ramping: These terms are used to describe the progressive increase or decrease of the infusion rate. Tapered infusion rates are often used with PN infusions that are administered over part of each day (e.g., 12 hours per day). Tapering at the beginning or end of the infusion allows a more gradual infusion, allowing the body to adjust to high glucose and electrolyte concentrations. The pump mathematically calculates the ramping rate once the duration of infusion and total volume to be infused are entered into the program.
- Timed infusion: This refers to an infusion governed by a 24-hour clock within the device. With timed infusion, the device must have a sufficient internal backup battery to maintain the clock accurately at all times. Timed infusions are used for ramping and tapering, automatic piggybacking, and intermittent dosing.
- Air-in-line: Designed to detect air in the line and may include air detection and air removal.
- Occlusion: Detects absence of fluid flowing upstream (between pump and the infusion container) or downstream (between the patient and the pump).
- Infusion complete: Alarm triggered by a preset volume limit (“infusion complete”). These alarms are helpful in preventing the fluid container from running dry because they can be set to sound before the entire solution container is infused.
- Low battery or low power: Gives the user ample warning of the pump's impending inability to function. A low-battery alarm means that the batteries need to be replaced or that an external power source needs to be connected. As a protective measure, when low-battery and low-power alarms are continued over a preset number of minutes, the pumps usually convert to a KVO rate. The preset KVO rate is usually between 0.1 and 5 mL.
- Nonfunctional or malfunctional: Alarm that means the pump is operating outside parameters and that the problem cannot be resolved. When this alarm sounds, the pump should be disconnected from the patient and returned to biomedical engineering or to the manufacturer for evaluation. The alert signifying a nonfunctional alarm may be worded in many ways, depending on the manufacturer.
- Not infusing: Indicates that all of the pump infusion parameters are not set. This feature prevents tampering or accidental setting changes. The pump must be programmed or changed and then told to “start.”
- Tubing: Ensures that the correct tubing has been loaded into the pump. If tubing is incorrectly loaded, this alarm will sound.
- Door: Indicates that the door that secures the tubing is not closed. Cassette pumps may give a “cassette” alarm if the cassette is unable to infuse within device operating parameters.
Occlusion Pressure and Occlusion Alarms
Electronic infusion pumps detect elevated pressures in the IV administration set between the infusion pump mechanism and the patient. When the sensor circuitry detects an elevated pressure that equals the pump occlusion alarm limit (e.g., 10 psi), the infusion pump will initiate an alarm and the pump will stop infusing. With many electronic infusion pumps, the occlusion pressure can be selected from low settings (e.g., 4 to 8 PSI) to high (e.g., 12 PSI). Occlusion alarms at low psi settings are common because the pumps are sensitive to even slight changes in pressure and very small IV catheter or patient movement. These devices are not, however, designed to detect infiltrations. Infiltration and extravasation pressures are typically much lower than pumps' downstream occlusion alarm limit settings and therefore will not trigger the occlusion alarm. Routine monitoring of the IV site or signs of infiltration/extravasation by the nurse is essential for patients no matter what time of infusion administration method is employed.
Infusion Pump Safety
Because better infusion pump design and engineering can reduce infusion pump problems, the FDA launched an initiative in 2010 to address safety issues related to infusion pumps. From 2005 through 2009, the FDA received approximately 56,000 reports of adverse events associated with the use of infusion pumps, including numerous injuries and deaths (FDA, 2010). These adverse event reports and associated device recalls have not been isolated to a specific manufacturer, type of infusion pump, or use environment; rather, they have occurred across the board. Causes of these adverse events include user errors and deficiencies in design and engineering, such as software defects and mechanical and electrical failures. The FDA provides guidance to the nurse to reduce infusion pump risks (Table 5-3).
| Plan ahead | Have a backup plan in case of an infusion pump failure that details:
Consider a secondary method of checking the expected volume infused, such as a time strip indicator or a Buretrol. |
| Label | Label the infusion pump channels with the name of the medication or fluid if your infusion pump does not display the name. Label the infusion pump tubing at the port of entry with the medication or fluid name. |
| Check | Verify that the infusion pump is programmed for the right dosage, at the right rate and volume to be infused. This is especially important at a change of shift, when any change is made to the infusion pump settings, when a new bag of medication/fluid is hung, or when new infusion tubing is primed. Obtain an independent double-check of infusion pump settings by a second clinician per your hospital/facility policy when infusing high-risk medications (e.g., insulin, heparin, vasopressors, propofol, total parenteral nutrition, morphine, etc.). An independent double-check involves two clinicians separately checking (alone and apart from each other, then comparing results) the infusion settings in accordance with the physician's order. |
| Monitor for signs of overinfusion or underinfusion of high-risk medications by using other patient monitoring systems, such as cardiac monitoring, pulse oximetry, end-tidal CO2measurement, and glucose meters, when applicable. Monitor the patient and infusion per your facility's protocol. | |
| Use | Use available resources, such as your Clinical/Biomedical Engineering Department, your area's “superusers,” and infusion pump instructions or troubleshooting guides when experiencing problems with an infusion pump. Use the drug library when applicable. Promptly respond and pay close attention to displayed alerts and cautions. Use the “five rights” for safe medication administration: the right patient, the right drug, the right dose, the right route, and the right time. |
| Report problem | Remove from use, tag with the specific problem and clinician contact information, and sequester any infusion pump that shows signs of breakage or damage, including small chips or cracks, if an unexplained alarm occurs or if the pump does not function as expected. Follow your hospital/facility protocol for reporting events where the infusion pump may have caused or contributed to a death or serious injury. You are also encouraged to report any other infusion pump safety concerns through your hospital/facility protocol. You are encouraged to file a voluntary report with the U.S. Food and Drug Administration (FDA) for any pump problem that you may encounter. Health Insurance Portability and Accountability Act of 1996 (HIPAA) restrictions do not apply to reports submitted to the FDA. |
Choosing a Flow-Control Device
A number of factors go into selecting the best device for any given infusion. Factors include prescribed infusion therapy, rate control requirements, infusion-related risks, patient care setting, and available resources within the organization (Gorski et al., 2021, p. S69). Lower risk infusions such as simple IV antibiotics or hydration fluids may be administered as gravity infusions or with a nonelectronic infusion pump, although electronic infusion pumps are widely used for most hospital-based infusions. For the many patients who are discharged home with infusion therapy needs, careful consideration is given to the safest method. Factors in the decision-making process include the type of infusion, the frequency of administration, infusion rate requirements, drug stability in solution, patient safety and lifestyle concerns, patient preference, and reimbursement (Gorski, 2017). Patient safety is maximized by teaching the patient and family how to administer the infusion therapy, how to use an infusion pump, how to identify potential problems, and when and whom to call with problems.
USE AND MANAGEMENT OF INFUSION EQUIPMENT
Focused Assessment
Key Nursing Interventions 1. Select and prepare the administration set and needed add-on devices. 2. Use filters when clinically indicated (e.g., blood, PN, lipids). 3. Inspect fluid containers, administration sets, and catheters for integrity before use. 4. Employ all necessary infection prevention interventions (ANTT, NC disinfection, protection of male Luer end of administration set with intermittent infusions, minimal use of add-on devices unless clinically indicated). 5. Follow the manufacturer's directions for the setup and maintenance of electronic infusion pumps and other flow-control devices and any other technology that is being used (e.g., visualization devices). 6. Set alarm limits on equipment as appropriate. 7. Monitor the patient and the infusion system for: a.Integrity of the infusion equipment. b.Patient's ability to safely move (i.e., transfer/ambulate) with a running infusion. c.Alarms: Respond in a timely manner. 8. Change solution container, administration set, and add-on device in accordance with INS Standards. |