Neurons are the basic elements of all rapid signal processing within the body. A neuron consists of a cell body, also called the soma, dendrites, and the nerve fiber, also called the axon (Figs. 3-1 and 3-2 ).
Classification of Afferent Nerve Fibers
Nerve fibers are called afferent if they transmit impulses from peripheral receptors to the central nervous system (CNS) and efferent if they transmit impulses from the CNS to the periphery. Afferent nerve fibers are classified as A, B, and C on the basis of fiber diameter and velocity of conduction of nerve impulses (Table 3-1).
Myelin that surrounds type A and B nerve fibers acts as an insulator that prevents flow of ions across nerve membranes. Type C fibers are unmyelinated. The myelin sheath is interrupted approximately every 1 to 2 mm by the nodes of Ranvier (see Fig. 3-1).
This successive excitation of nodes of Ranvier by an action potential that jumps between successive nodes is termed saltatory conduction. Saltatory conduction allows for a 10-fold increase in the velocity of nerve transmission.
Evaluation of Peripheral Nerve Function
Nerve conduction studies are useful in the localization and assessment of peripheral nerve dysfunction.
Electromyographic testing is helpful in determining the etiology of neurologic dysfunction that may occur after surgery.
Action Potential
Electrical potentials exist across nearly all cell membranes, reflecting principally the difference in transmembrane concentrations of sodium and potassium ions.
The resulting voltage difference across the cell membrane is called the resting membrane potential. The cytoplasm is electrically negative (typically -60 to -80 mV) relative to the extracellular fluid (Fig. 3-3).
An action potential is the rapid change in transmembrane potential due to the opening of sodium channels (depolarization) and rapid influx of sodium ions down the concentration gradient, reversing the net negative charge within the cell. The membrane resting potential is restored by the closing of the sodium channels and the opening of potassium channels (repolarization) after the action potential has passed.
Propagation of action potentials along the entire length of a nerve axon is the basis of rapid signal transmission along nerve cells. The size and shape of the action potential varies among excitable tissues (see Fig. 3-3).
Abnormal Action Potentials
A deficiency of calcium ions in the extracellular fluid (hypocalcemia) prevents the sodium channels from closing between action potentials (tetany).
Low potassium ion concentrations in extracellular fluid increase the negativity of the resting membrane potential, resulting in hyperpolarization and decreased cell membrane excitability.
Local anesthetics decrease permeability of nerve cell membranes to sodium ions, preventing achievement of a threshold potential that is necessary for generation of an action potential.
Neurotransmitters and Receptors
Neurotransmitters are chemical mediators that are released into the synaptic cleft in response to the arrival of an action potential at the nerve ending. Neurotransmitter release is voltage dependent and requires the influx of calcium ions into the presynaptic terminals (see Fig. 3-2).
Neurotransmitters may be excitatory or inhibitory, depending on the ion selectivity of the protein receptor (Table 3-2).
Volatile anesthetics produce a broad spectrum of actions, as reflected by their ability to modify both inhibitory and excitatory neurotransmission at presynaptic and postsynaptic loci within the CNS. The precise mechanism of these effects remains uncertain. It is likely that volatile anesthetics interact with multiple neurotransmitter systems by a variety of mechanisms.
G Protein-coupled Receptors (Fig. 3-4). The recognition site faces the exterior of the cell membrane to facilitate access of water-soluble endogenous ligands and exogenous drugs, whereas the catalytic site faces the interior of the cell.
G
 proteins can either be stimulatory, promoting a specific enzymatic reaction within the cell, or inhibitory, depressing a specific enzymatic reaction.
proteins can either be stimulatory, promoting a specific enzymatic reaction within the cell, or inhibitory, depressing a specific enzymatic reaction.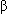 -adrenergic receptors couple with stimulatory G
-adrenergic receptors couple with stimulatory G s proteins and increase the activity of adenylyl cyclase.
s proteins and increase the activity of adenylyl cyclase.Opioid receptors are associated with inhibitory G
 i proteins that decrease the activity of adenylyl cyclase.
i proteins that decrease the activity of adenylyl cyclase.By regulating the level of activity of adenylyl cyclase, the
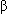 -adrenergic and opioid receptors modulate the internal level of cyclic adenosine monophosphate (cAMP), which functions as an intercellular second messenger (see Fig. 3-4).
-adrenergic and opioid receptors modulate the internal level of cyclic adenosine monophosphate (cAMP), which functions as an intercellular second messenger (see Fig. 3-4).
Many hormones and drugs act through G protein-coupled receptors, including catecholamines, opioids, anticholinergics, and antihistamines.
Dopamine represents more than 50% of the CNS content of catecholamines, with high concentrations in the basal ganglia. Dopamine can be either inhibitory or excitatory, depending on the specific dopaminergic receptor that it activates. Dopamine is important to the reward centers of the brain and plays a key role in addiction and drugs.
Norepinephrine is present in large amounts in the reticular activating system and the hypothalamus, where it plays a key role in natural sleep and analgesia.
Substance P is an excitatory neurotransmitter co-released by terminals of pain fibers that synapse in the substantia gelatinosa of the spinal cord.
Endorphins are endogenous opioid peptide agonists (act through the μ opioid receptor, the same receptor responsible for the effects of administered opioids).
Ion Channels. There are three basic types of ion channels: (a) ligand-gated ion channels ionotropic receptors, (b) voltage-sensitive ion channels, and (c) ion channels that respond to other types of gating.
Ligand-gated ion channels are complexes of protein subunits that act as switchable portals for ions (involved principally with fast synaptic transmission between excitable cells).
Excitatory ligand-gated ion channels cause the inside of the cell to become less negative typically by facilitating the influx of cations into the cell (acetylcholine, glutamate, serotonin).
Inhibitory ligand-gated ion channels cause the inside of the cell to become less negative, typically by facilitating the flux of chloride into the cell. Potassium channels that facilitate the efflux of potassium ions are also inhibitory (
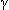 -amino butyric acid [GABA]), glycine).
-amino butyric acid [GABA]), glycine).
Voltage-gated ion channels are complexes of protein subunits that act as switchable portals sensitive to membrane potential through which ions can pass through the cell membrane (open and close in response to changes in voltage across cell membranes).
Voltage-gated sodium channels are the site of local anesthetic action (local anesthetics block neural conduction by blocking passage of sodium through the voltage-gated sodium channel).
The human ether-a-go-go related gene (hERG) potassium channel is sensitive to many drugs and is responsible for sudden death from drugs that predispose the patient to torsades de pointes (inhibition of the hERG potassium channel is the reason for the black box warning on droperidol).
Receptor Concentration
Receptors in cell membranes are not static components of cells.
Excess circulating concentrations of ligand often results in a decrease in the density of the target receptors in cell membranes (excessive circulating norepinephrine in patients with pheochromocytoma leads to downregulation of
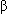 -adrenergic receptors).
-adrenergic receptors).
The Synapse
Structure
The synapse functions as a diode that transmits an action potential from the presynaptic membrane to the postsynaptic membrane across the synaptic cleft (Fig. 3-5).
Calcium triggers the fusion of the vesicle to the cell membrane and the release of the neurotransmitter into the synaptic cleft through exocytosis, resulting in the extrusion of the contents of the synaptic vesicles.
Synaptic Modulation. The resting transmembrane potential of neurons in the CNS is about -70 mV, less than the -90 mV in large peripheral nerve fibers and skeletal muscles.
Synaptic delay reflects the time for release of the neurotransmitter from the synaptic varicosity, diffusion of the neurotransmitter to the postsynaptic receptor, and the subsequent change in permeability of the postsynaptic membrane to various ions.
Synaptic fatigue is a decrease in the number of discharges by the postsynaptic membrane when excitatory synapses are repetitively and rapidly stimulated (decreases excessive excitability of the brain as may accompany a seizure, thus acting as a protective mechanism against excessive neuronal activity).
The mechanism of synaptic fatigue is presumed to be exhaustion of the stores of neurotransmitter in the synaptic vesicles.
Synaptic fatigue is unmasked at the neuromuscular junction in myasthenia gravis when the enormous reserve for neuromuscular transmission is limited by either pre- or postsynaptic autoimmune damage.
Factors that Influence Neuron Responsiveness. Neurons are highly sensitive to changes in the pH of the surrounding interstitial fluids (alkalosis enhances neuron excitability and acidosis depresses neuron excitability).