- Binds to
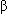 -tubulin subunits on microtubules; this action blocks cells in mitosis, leading to cell death.
-tubulin subunits on microtubules; this action blocks cells in mitosis, leading to cell death. - Also has antiangiogenic activity.
- Decreased spread of breast cancer.
Contraindicated in:
Use Cautiously in:
CV: chest pain, edema, left ventricular dysfunction, myocardial ischemia.
Derm: alopecia, hyperpigmentation, nail disorder, palmar-plantar erythrodysesthesia (combination therapy with capecitabine), exfoliation, hot flushes, pruritus, rash.
EENT: ↑ lacrimation.
GI: abdominal pain, anorexia, constipation, diarrhea, mucositis, nausea, stomatitis, vomiting, altered taste.
GU: ↓ fertility.
Hemat: myelosuppression.
MS: arthralgia, musculoskeletal pain, myalgia.
Neuro: fatigue, peripheral neuropathy, weakness, dizziness, headache, insomnia.
Resp: dyspnea.
Misc: hypersensitivity reactions.
Drug-Drug:
Drug-Natural Products:
Drug-Food:
Hepatic Impairment
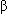 -tubulin subunits on microtubules; this action blocks cells in mitosis, leading to cell death.
-tubulin subunits on microtubules; this action blocks cells in mitosis, leading to cell death.Absorption: IV administration results in complete bioavailablity.
Distribution: Unknown.
Metabolism/Excretion: Extensively metabolized by the liver, primarily by the CYP3A4 isoenzyme. Metabolites are not active and are excreted mainly by the kidneys.
Half-life: 52 hr.
NDC Code*