AUTHORS: Omar Karim, BS and Caroline P. Meehan, MD
Gout refers to a group of disease states caused by deposition of monosodium urate (MSU) in tissue, resulting from prolonged hyperuricemia. Clinical manifestations of gout include acute and chronic arthritis, soft tissue inflammation, tophus formation, gouty nephropathy, and nephrolithiasis. Untreated hyperuricemia in patients with gout may lead to chronic destructive deforming arthritis.
| ||||||||||||||||||||||||||||||||
Self-reported prevalence in the U.S. is estimated at 3.9% of adults.1 In the U.S., gout has been diagnosed in more than 10 million adults.2
Male:female ratio ∼4:1. However the prevalence of gout among women has increased in past decade. Compared with men, gout in women is more likely to be associated with diabetes, coronary heart disease, and chronic kidney disease.3
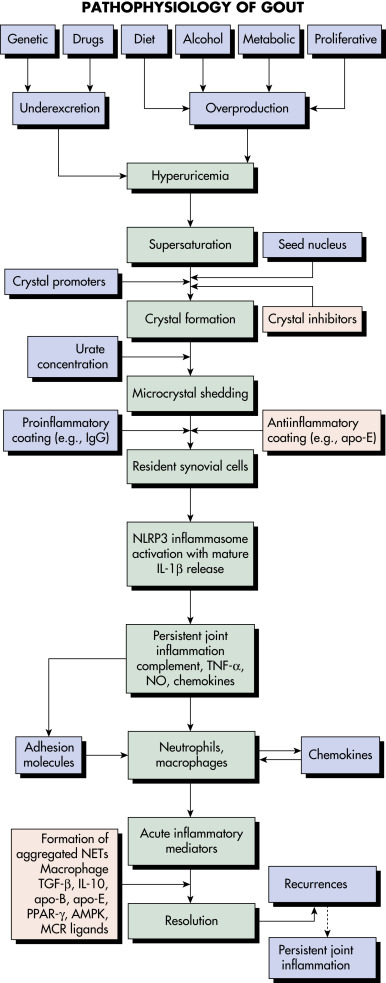
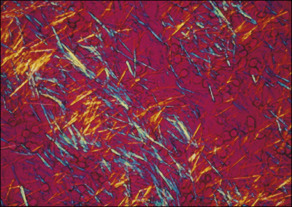
- Gout is caused by inflammation resulting from MSU crystal deposition. The primary risk factors for MSU deposition are hyperuricemia, although local factors such as temperature, pH, and mechanical stress may play a role. Figs. E1, E2, and E3 illustrate the pathophysiology of gout.
- Hyperuricemia and gout develop from excessive uric acid production, a decrease in the renal excretion of uric acid, or both.
- Primary hyperuricemia results from an inborn error of metabolism and may be attributed to several biochemical defects.
- Secondary hyperuricemia may develop as a complication of acquired disorders (e.g., leukemia) or as a result of the use of certain drugs (e.g., diuretics). Consumption of alcohol, especially beer, increases the risk of gout, and fructose-rich beverage intake is associated with hyperuricemia. Gout promoters and inhibitors are summarized in Table 1.
TABLE 1 Gout Promoters and Inhibitors∗
| Crystal formation | Seed nucleus (particulate) Immunoglobulin Phagocytes Low temperature Low pH Cation concentration Intraarticular dehydration Other (unknown) macromolecules | ||
| Triggering the acute flare (local factors) | Rapid change in urate level Microcrystal release IgG coat (apolipoproteins B, E inhibitory) Complement activation (classical, alternate, MAC) Inflammasome activation Cytokine and chemokine release Endothelial activation (e-selectin, ICAM-1, VCAM-1) Local trauma | ||
| Presence of susceptible phagocytes, mast cells (systemic events) | Surgery, trauma Infections, other intercurrent systemic illness Alcohol, dietary intake Drugs that raise or lower circulating urate level |
ICAM-1, Intercellular adhesion molecule 1; Ig, immunoglobulin; MAC, membrane attack complex; VCAM-1, vascular cell adhesion molecule 1.
∗A diverse array of proteins and other mediators have been identified on the surfaces of urate crystals. In addition to their proinflammatory effect through opsonizing existing crystals, immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies may promote crystal formation by providing a stable molecular platform for crystal nucleation and growth. Apolipoproteins are antiinflammatory molecules that coat crystals. The characteristics of the phagocytes encountering the crystals may be crucial; macrophages that are more differentiated are less likely to elicit proinflammatory cytokines.
From Hochberg MC et al: Rheumatology, ed 7, Philadelphia, 2019, Elsevier.
Figure E1 Many signals impact the critical points in this pathway.

Patients with hyperuricemia do not necessarily form urate crystals. Crystals ingested by phagocytes sometimes elicit remarkably little inflammation, and the inflammation is often low grade or clinically silent in advanced gout, indicating robust regulation of the pathways shown. Factors leading to the spontaneous termination of the acute gout flare are incompletely understood. AMPK, Adenosine monophosphate-activated protein kinase; apo, apolipoprotein; IgG, immunoglobulin G; IL, interleukin; MCR, melanocortin receptor; NET, neutrophil extracellular trap; NLRP3, nucleotide-binding oligomerization domain-like receptor 3; NO, nitric oxide; PPAR, peroxisome proliferator-activated receptor; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α.
From Hochberg MC: Rheumatology, ed 7, Philadelphia, 2019, Elsevier.
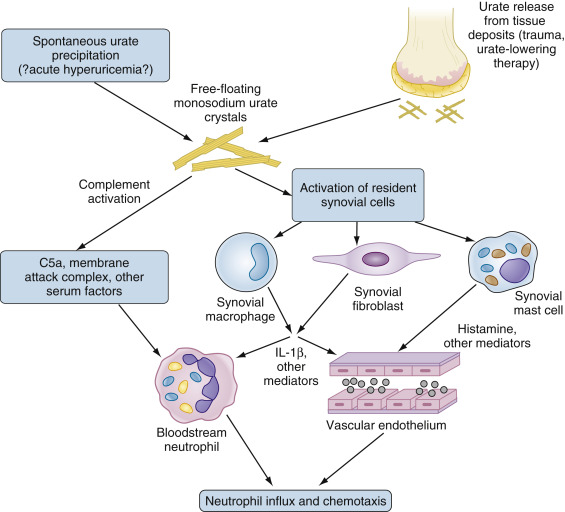
Figure E2 Initial phases of monosodium urate (MSU) crystal-induced activation.
IL, Interleukin.
The presence of “fresh” MSU crystals, resulting either from spontaneous precipitation or the liberation of crystals from established pools, results in direct complement activation and activation of resident cells in the synovium including macrophages, fibroblasts, and mast cells. Activated cells produce IL-1β and other cytokines, as well as multiple other mediators (not all illustrated) that in turn activate both bloodstream neutrophils and endothelial cells. These responses permit neutrophils to adhere to and traverse the endothelium, resulting in neutrophil influx and the further propagation of inflammation as neutrophils undergo direct activation by MSU crystals (not illustrated). See text for additional details.
From Firestein GS et al: Firestein & Kelley’s textbook of rheumatology, ed 11, Philadelphia 2021, Elsevier.
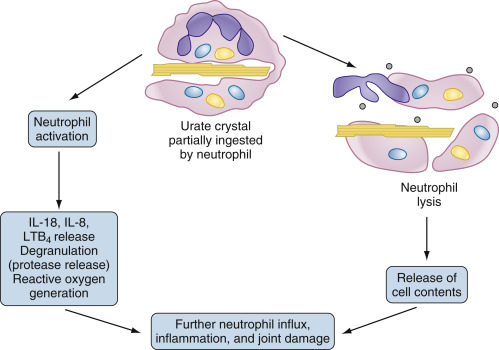
Figure E3 Propagation of the acute gouty response by activated neutrophils.

Neutrophils that enter the joint migrate toward and phagocytose crystals. In the case of crystals coated with immunoglobulins and complement, the resultant activation results in synthesis and/or release of inflammatory mediators such as interleukin-1β (IL-1β), IL-8, and tumor necrosis factor, as well as proteases and reactive oxygen species. In the case of uncoated crystals, the crystal frequently interacts with and lyses the membrane of the phagolysosome, spilling toxic contents and leading to cell lysis. In both cases, the result is local tissue damage and recruitment of additional neutrophils from the bloodstream in an explosive inflammatory cycle. LTB4, Leukotriene B4.
From Firestein GS et al: Firestein & Kelley’s textbook of rheumatology, ed 11, Philadelphia 2021, Elsevier.
- Rapid onset of pain and swelling and erythema of a distal joint and/or periarticular soft tissue. Box 1 summarizes clinical pearls in acute gout attacks.
- May present as monoarthritis of any joint. Acute gout of the first metatarsophalangeal (MTP) joint is known as podagra.
- 10% to 15% of attacks are polyarticular.
- Spontaneous resolution occurs over days to weeks.
BOX 1 Acute Gout: Clinical Pearls
From Hochberg MC et al: Rheumatology, ed 7, Philadelphia, 2019, Elsevier.
- Insidious onset of painless arthritis and soft tissue swelling
- Distal small joints characteristic
- May be confused with nodal osteoarthritis
- Box 2 summarizes clinical pearls in chronic gout
BOX 2 Chronic Gout: Clinical Pearls
| |||||
From Hochberg MC et al: Rheumatology, ed 7, Philadelphia, 2019, Elsevier.
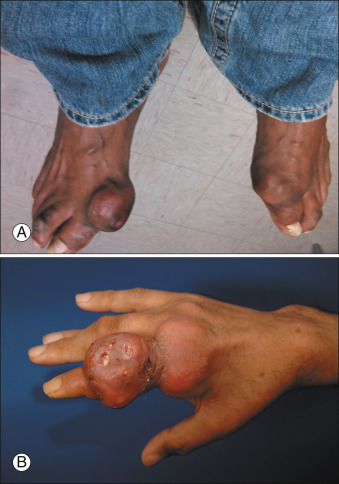
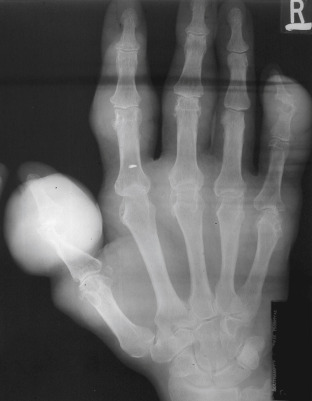
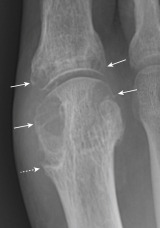
Figure E4 Advanced tophaceous gout with multiple intra-articular and periarticular tophi.
Tophi observed on bilateral first metatarsophalangeal joints (A) (right greater than left) and the hand (B) in a patient with severe tophaceous gout.
From Hochberg MC et al: Rheumatology, ed 7, Philadelphia, 2019, Elsevier.