Specific Entities
- Microcornea: Horizontal corneal diameter small for age. May be isolated or associated with microphthalmia, cataract, or nanophthalmos.
- Posterior embryotoxon: A prominent, anteriorly displaced Schwalbe line. Higher risk for the development of early-onset glaucoma. May be normal or seen in association with Axenfeld–Rieger and Alagille syndromes.
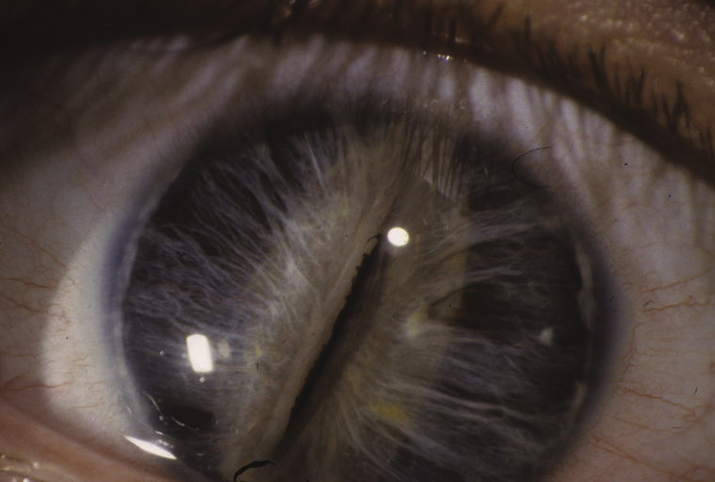
- Axenfeld–Rieger spectrum: Ranges from posterior embryotoxon and iris strands inserting onto Schwalbe line or the cornea to more severe iris malformations including polycoria and corectopia. Glaucoma develops in 50% to 60% of patients. Usually autosomal dominant mutations of PITX2 and FOXC1, although others have been implicated. May be associated with abnormal teeth (e.g., microdontia, conical teeth, hypodontia), skeletal abnormalities, and redundancy of the periumbilical skin. Growth hormone deficiency, cardiac defects, deafness, and mental retardation may be seen (see Figure 8.12.1).
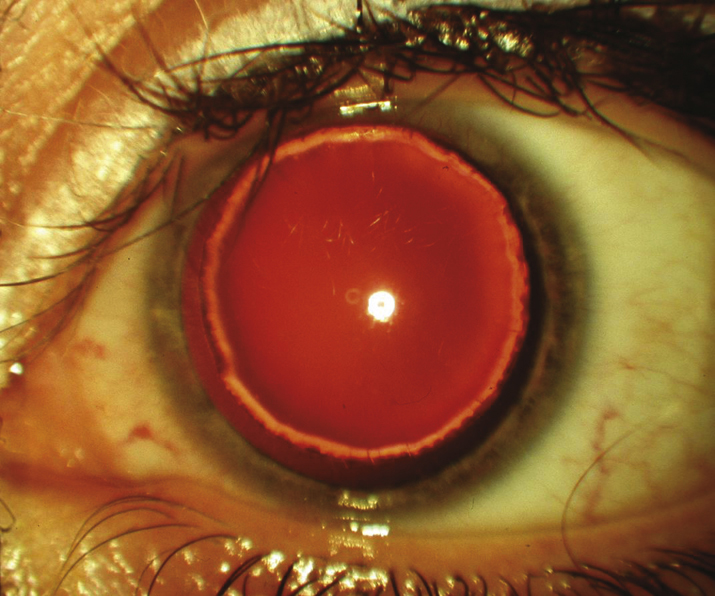
- Peters anomaly: Failure of the lens to form properly from the surface ectoderm and completely detach from surface epithelium during 4 to 7 weeks gestation. Central corneal opacity, usually with iris strands that extend from the collarette to a posterior corneal defect behind the scar. The lens may be clear and normally positioned, cataractous and displaced anteriorly (making the anterior chamber shallow), or adherent to the corneal defect. “Peters plus” syndrome is characterized by an associated skeletal dysplasia with short stature and is more common in bilateral cases. Other malformations may also be seen (see Figure 8.12.2).
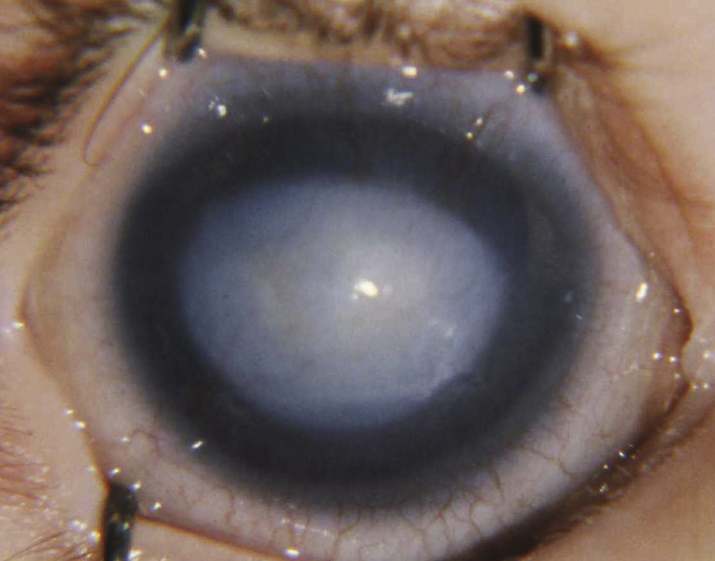
- Microspherophakia: The lens is small and spherical in configuration. It can subluxate into the anterior chamber, causing a secondary glaucoma. Can be isolated or seen in association with Weill–Marchesani syndrome (see Figure 8.12.3).
- Anterior and posterior lenticonus: An anterior or posterior ectasia of the lens surface, posterior occurring more commonly than anterior. Often associated with cataract. Unilateral or bilateral. Anterior lenticonus is associated with Alport syndrome. Posterior lenticonus is usually isolated but may be autosomal dominant. It can also be seen with Alport syndrome.
- Ectopia lentis: See 13.10, SUBLUXED OR DISLOCATED CRYSTALLINE LENS.
- Ectopia lentis et pupillae: Lens displacement associated with pupillary displacement in the opposite direction. Usually not associated with glaucoma.
- Aniridia: Bilateral, near-total absence of the iris. Glaucoma, macular hypoplasia with poor vision, nystagmus, refractive error, and corneal pannus are common. Aniridia is autosomal dominant in two-thirds of patients, a type usually without systemic implications. It occurs sporadically in one-third of cases. May be part of a panocular disorder due to mutations in the master control gene of the eye, PAX6. If PAX6 is deleted as part of a larger chromosomal deletion, it is called WAGR syndrome (Wilms tumor, aniridia, genital abnormalities, retardation). Children with sporadic aniridia have about a 30% chance of Wilms tumor development and require screening, typically with renal ultrasound.
- Sclerocornea: Nonprogressive scleralization of the cornea. Unilateral or bilateral. May be mild and peripheral or severe and diffuse. Associated with severe anterior segment dysgenesis and risk for glaucoma. Often associated with microphthalmia.
- Primary aphakia: Failure of lens development. Usually associated with microphthalmia and severe intraocular dysgenesis including retinal dysplasia and corneal opacity. High risk for glaucoma.