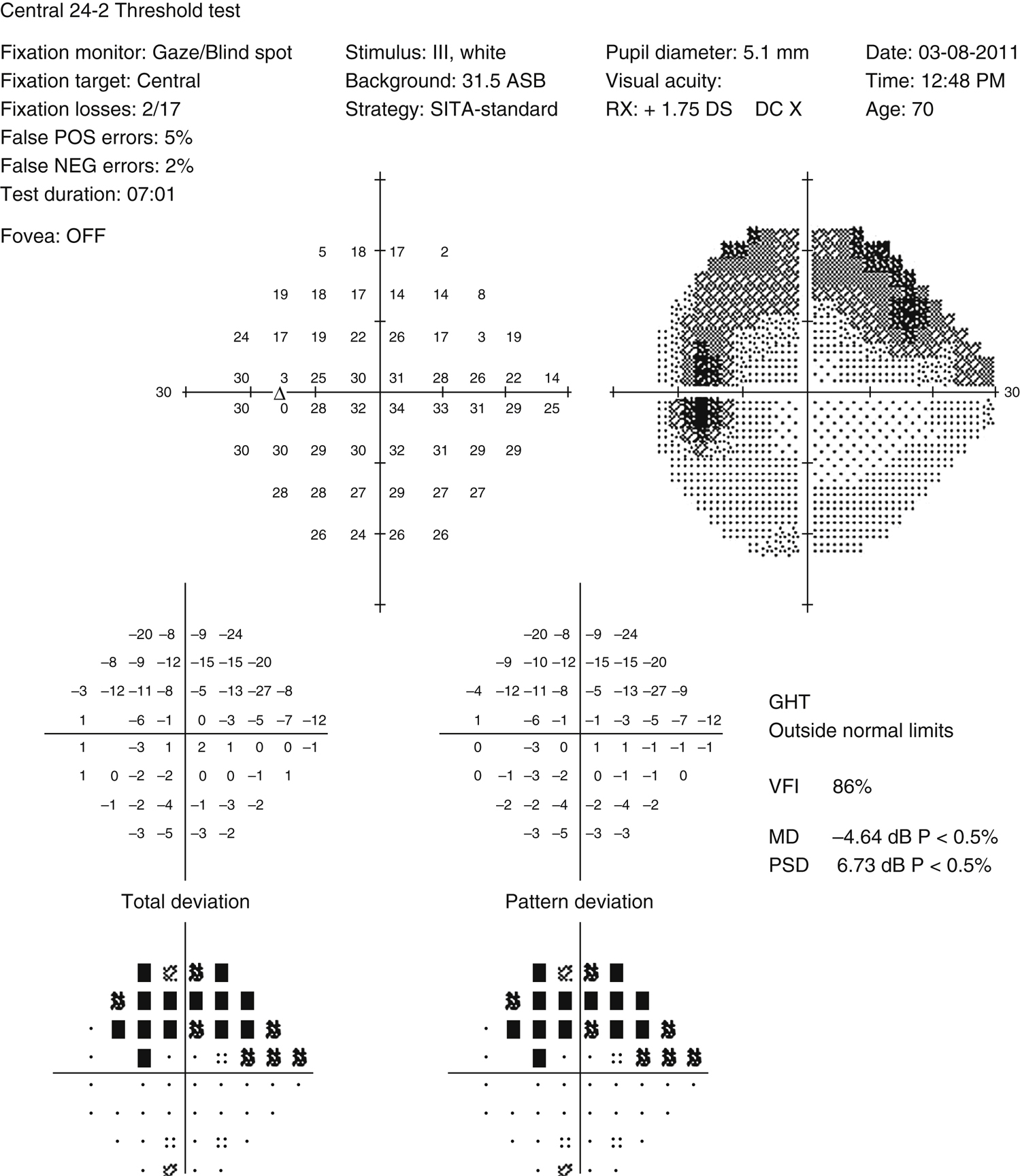
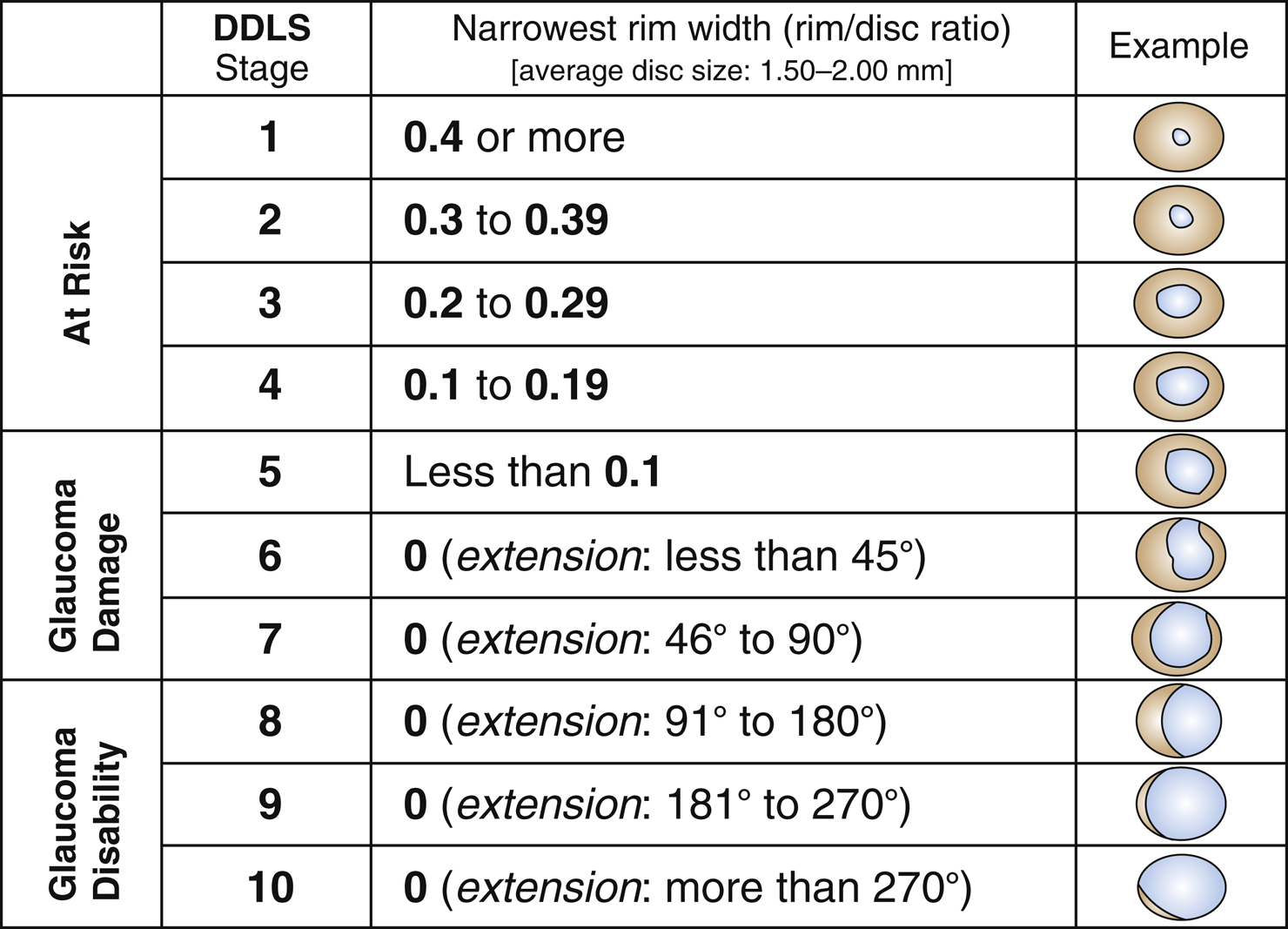
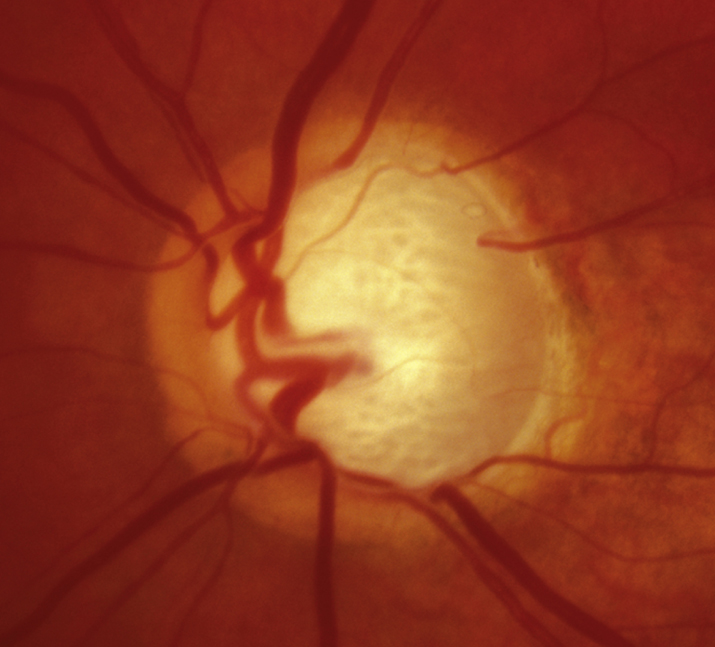
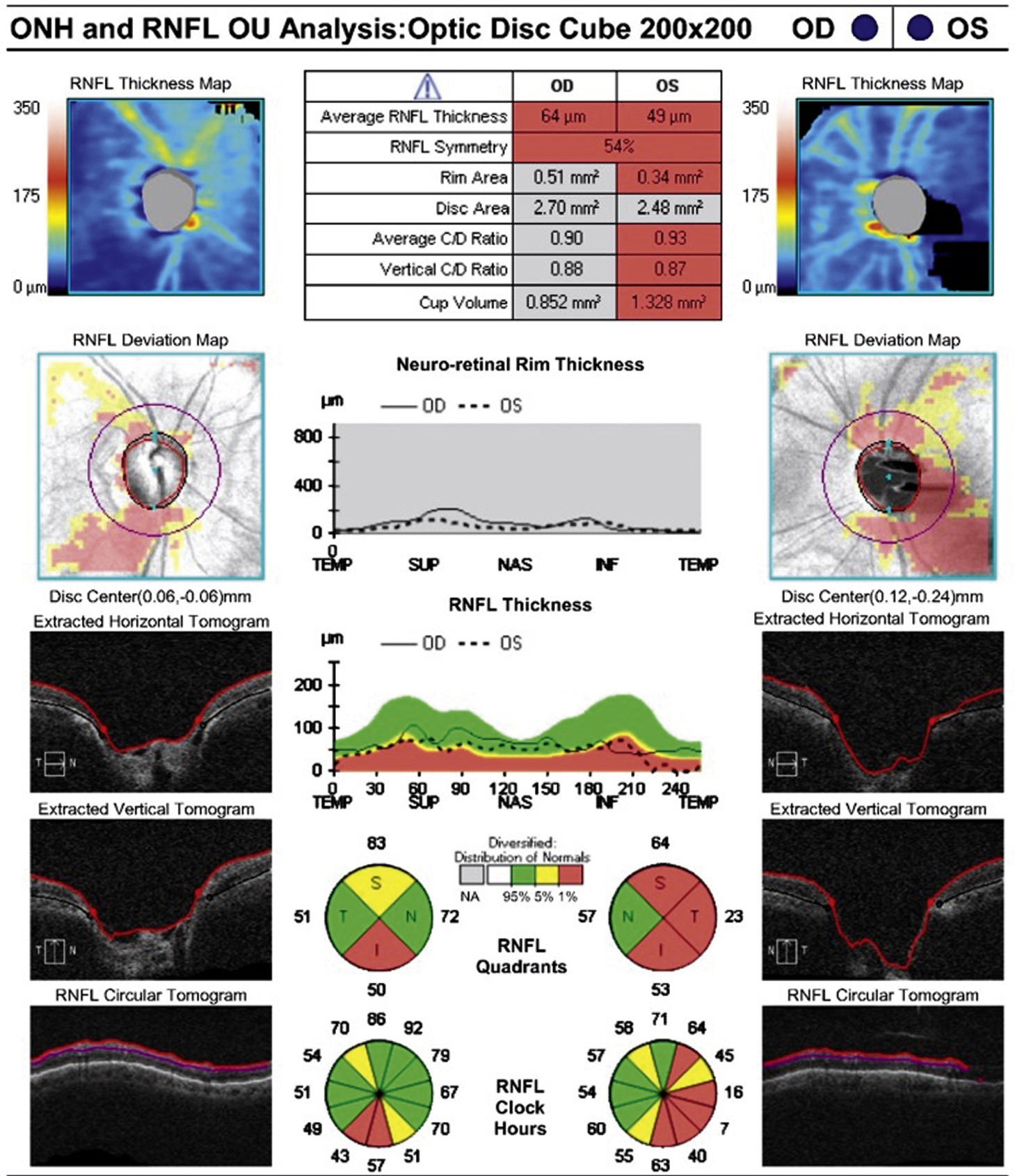
Usually asymptomatic until the later stages. Symptoms may include visual field defects. Usually bilateral, but can present asymmetrically. Severe field damage and loss of central fixation typically do not occur until late in the disease.
If anterior chamber angle open on gonioscopy:
- Ocular hypertension: Normal optic nerve and visual field. See 9.3, OCULAR HYPERTENSION.
- Physiologic optic nerve cupping: Static enlarged C/D ratio without rim notching or visual field loss. Usually normal IOP and large optic nerve (>about 2 mm). Often familial.
- Secondary open-angle glaucoma: Identifiable cause for open-angle glaucoma including inflammatory, exfoliative, pigmentary, steroid-induced, angle recession, traumatic (as a result of direct injury, blood, or debris), and glaucoma related to increased episcleral venous pressure (e.g., Sturge–Weber syndrome, carotid–cavernous fistula), intraocular tumors, degenerated red blood cells (ghost cell glaucoma), lens-induced, degenerated photoreceptor outer segments following chronic rhegmatogenous retinal detachment (Schwartz–Matsuo syndrome), or developmental anterior segment abnormalities.
- Low-tension glaucoma: Same as primary open-angle glaucoma (POAG) except normal IOP. See 9.2, LOW-TENSION PRIMARY OPEN-ANGLE GLAUCOMA (NORMAL PRESSURE GLAUCOMA).
- Previous glaucomatous damage (e.g., from steroids, uveitis, glaucomatocyclitic crisis, trauma) in which the inciting agent has been removed. Nerve appearance now static.
- Optic atrophy: Characterized by disproportionally more optic nerve pallor than cupping. IOP usually normal unless a secondary or unrelated glaucoma is present. Color vision and central vision are often decreased, although not always. Causes include tumors of the optic nerve, chiasm, or tract; syphilis, ischemic optic neuropathy, drugs, retinal vascular or degenerative disease, and others. Visual field defects that respect the vertical midline are typical of intracranial lesions localized at the chiasm or posterior to it.
- Congenital optic nerve defects (e.g., tilted discs, colobomas, optic nerve pits): Visual field defects may be present but are static.
- Optic nerve drusen: Optic nerves not usually cupped and drusen often visible. Visual field defects may remain stable or progress unrelated to IOP. The most frequent defects include arcuate defects or an enlarged blind spot. Characteristic calcified lesions can be seen on B-scan ultrasonography (US) (as well as on computed tomography [CT]). Autofluorescence can also highlight nerve drusen.
If anterior chamber angle closed or partially closed on gonioscopy:
- Chronic angle closure glaucoma (CACG): Shallow anterior chamber. May present with history of episodic blurred vision or headache. PAS present on gonioscopy. See 9.5, CHRONIC ANGLE CLOSURE GLAUCOMA.
Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open angle glaucoma. Arch Ophthalmol. 2002;120(6):701-713.