Variable onset of central visual loss, central or paracentral scotoma, metamorphopsia, photopsias in the central visual field.
(See Figures 11.17.1 and 11.17.2.)
Figure 11.17.1: Exudative AMD.
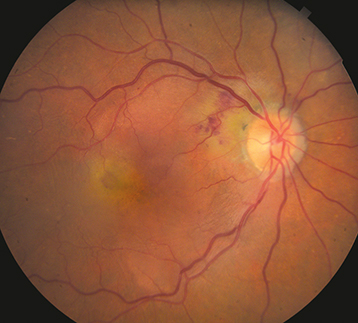
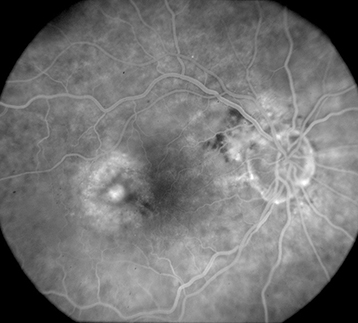
Figure 11.17.2: Intravenous fluorescein angiography of exudative AMD.
Critical
Drusen and SRF, ME or RPE detachment associated with CNV.
Other
Subretinal or intraretinal blood. Retinal exudates, subretinal fibrosis (disciform scar). Retinal angiomatous proliferation (RAP) is an intraretinal variant of neovascular AMD and is characterized by focal telangiectatic retinal vessels with an adjacent superficial retinal hemorrhage and associated intraretinal edema and RPE detachment. Some neovascular AMD patients may present with VH.
Idiopathic polypoidal choroidal vasculopathy (IPCV): Multiple serosanguineous macular and RPE detachments. ICGA highlights characteristic choroidal polyp-like aneurysmal dilations most often located in the peripapillary region. This is considered a variant of neovascular AMD and is more common in those of Asian and African descent. See 11.18, Idiopathic Polypoidal Choroidal Vasculopathy.
Risk Factors for Loss of Vision
Advanced age, hyperopia, light-colored iris, family history, soft (large) drusen, focal subretinal pigment clumping, RPE detachments, systemic HTN, and smoking. Note that patients with wet AMD in one eye have a 10% to 12% risk per year of developing CNV in the fellow eye. The risk increases for eyes with multiple or confluent soft drusen with RPE clumping.
Ocular histoplasmosis syndrome: Small white–yellow chorioretinal scars and peripapillary atrophy. May also present with CNV. See 11.24, Ocular Histoplasmosis.
Angioid streaks: Bilateral subretinal red-brown or gray irregular bands often radiating from the optic disc. See 11.23, Angioid Streaks.
High myopia: Significant myopic refractive error, lacquer cracks, tilted disc. See 11.22, Pathologic/Degenerative Myopia.
Other CNV-predisposing conditions include drusen of the optic nerve, choroidal rupture, choroidal tumors, photocoagulation scars, inflammatory focal chorioretinal spots, and idiopathic causes.
Types of Neovascular AMD Lesions
Occult CNV (Type 1): Ill-defined, stippled, flat, or elevated subtle late leakage on IVFA and located in the sub-RPE location by OCT.
Classic CNV (Type 2): Early-phase IVFA demonstrates a well-delineated area of lacy hyperfluorescence with prominent leakage in later phases and located in the subneurosensory retinal location by OCT.
RAP (Type 3): Focal intraretinal hyperfluorescence on IVFA and ICGA. High-speed ICGA is particularly sensitive and may show characteristic “hair pin loop” with retinal feeder and draining vessels. OCTA shows this focal form of neovascularization to be intraretinal.
Depends on the treatment algorithm used, but typically monthly follow-up until the CNV lesion is inactive with resolution of exudative signs based on examination and OCT. Patients receiving anti-VEGF therapy need indefinite follow-up, though the follow-up frequency depends on treatment response and treatment algorithm, for example, PRN versus TAE. Patients receiving intravitreal injections should be given warning symptoms for endophthalmitis and RD.
DugelPU, KohA, OguraY, et al; HAWK and HARRIER Study Investigators. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72–84.
HeierJS, BrownDM, ChongV. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548.
KhananiAM, KotechaA, ChangA, et al; TENAYA and LUCERNE Investigators. TENAYA and LUCERNE: Two-year results from the phase 3 neovascular age-related macular degeneration trials of faricimab with treat-and-extend dosing in year 2. Ophthalmology. 2024:131(8):914–926.
LanzettaP, KorobelnikJF, HeierJS, et al; PULSAR Investigators. Intravitreal aflibercept 8 mg in neovascular age-related macular degeneration (PULSAR): 48-week results from a randomised, double-masked, non-inferiority, phase 3 trial. Lancet. 2024:403(10432):1141–1152.
RosenfeldPJ, BrownDM, HeierJS, et al; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431.
The CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908.

