Description
- Lusitropy describes the ability of the myocardium to relax following contraction, hence the ventricle's diastolic function.
- Maintaining cardiac output is a direct function of normal contraction (inotropy) relaxation (lusitropy) properties.
- Myocardial contraction: Electrical depolarization activates the voltage-gated sodium channels triggers a rapid release of calcium from the sarcoplasmic reticulum. The initial burst of calcium release is inhibited by ryanodine (discontinues sarcoplasmic calcium release into the intracellular space). Calcium is a principal controlling mechanism of actin–myosin interaction.
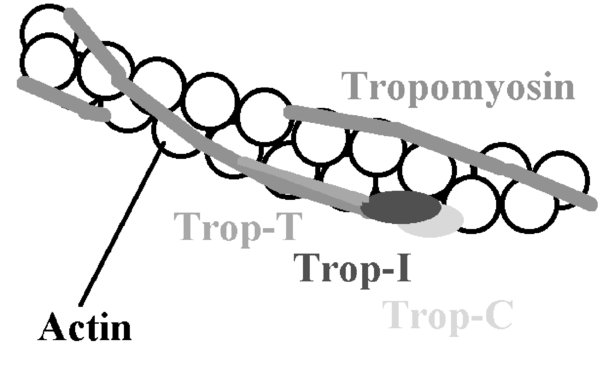
Calcium binds to troponin C which then allosterically modulates tropomyosin; this results in uncovering of actin binding sites. When uncovered, myosin heads can bind hydrolyze ATP (converts the chemical energy in ATP into mechanical energy; ATP
 ADP + phosphate) to drive filament sliding; known as a cycle. Repeated cycles result in the movement of myosin heads along actin filaments, resulting in contraction.
ADP + phosphate) to drive filament sliding; known as a cycle. Repeated cycles result in the movement of myosin heads along actin filaments, resulting in contraction.FIGURE 1. Tropomyosin chains cover actin binding sites.
When calcium binds to troponin C, tropomyosin uncovers or “exposes” the actin binding sites which allows myosin to bind.
- Myocardial relaxation, hence, results from the removal sequestration of intracellular calcium. A decrease in calcium concentration results in decreased binding to troponin C tropomyosin “re-covering” the actin binding sites, myosin “unbinding.” Calcium removal is performed by the:
Positive lusitropy describes increased relaxation.
- Beta-agonists increase contractility/inotropy/chronotropy are also important in improving relaxation via improved calcium ion uptake. This allows for subsequent normal filling preload, improved cardiac output.
- At a molecular level, beta-agonism decreases the amount of calcium available for binding with troponin C by phosphorylating the phospholamban molecule. Normally, non-phosphorylated phospholamban is a SERCA antagonist, decreases intracellular calcium removal.
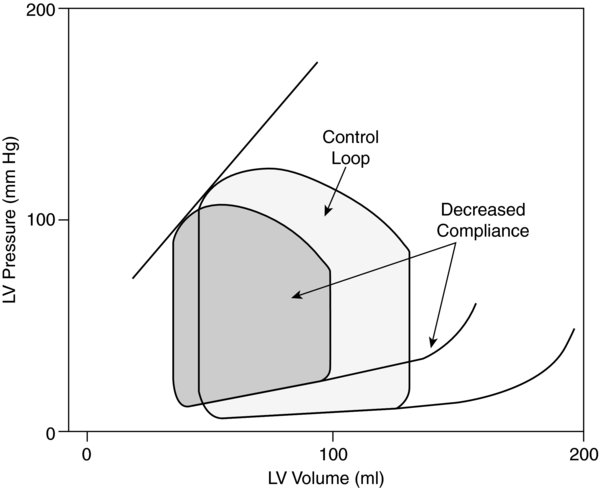
FIGURE 2. Effects of left ventricular diastolic failur caused by decreased ventricular compliance (e.g., hypertrophy) on left ventricular pressure-volume loop.
Heart rate, inotropy systemic vascular resistance are unchanged.
- Negative lusitropy describes decreased relaxation involves:
- Intracellular acidosis may inhibit calcium reuptake by the sarcoplasmic reticulum.
Physiology/Pathophysiology
- Diastolic dysfunction, or negative lusitropy, results in impaired ventricular compliance.
- On a molecular level, it is related to an imbalance in the distribution of phospholamban SERCA. The increased levels of intracellular calcium result in impaired lusitropy.
- Increased left ventricular end-diastolic pressures (LVEDP) will decrease preload (LVEDV), with resultant decreases in stroke volume cardiac output
- Over time, left atrial pulmonary arterial pressures can increase (this can result in elevated right ventricular afterload)
- Coronary perfusion pressure (CPP) also decreases secondary to increased intraventricular pressures during diastole (CPP = MAP – LVEDP, where MAP is mean arterial pressure).
- Diastolic dysfunction is an independent determinant of heart failure; poorer outcomes are seen when it is present early in post-acute myocardial dysfunction. It is diagnosed in 3 steps:
- Clinical (heart failure)
- Normal ejection fraction
- Increased diastolic filling pressures
- Echocardiographic indices:
- Isovolumic relaxation time (normal 70 ms) is the time interval between aortic valve closure mitral valve opening. When prolonged, it indicates poor myocardial relaxation.
- Transmitral inflow (E/A ratio): The “E” wave represents flow during the rapid filling phase of ventricular diastole (after mitral valve opening); it represents the left atrial to left ventricular gradient. The “A” wave represents flow during atrial contraction is approximately one-third to one-half the size of the E wave.
- During impaired relaxation, the E wave decreases in size (decreased left atrial-left ventricular gradient), the A wave increases in size (increased contribution), the E/A ratio is 1.
- As impaired ventricular relaxation progresses, left atrial pressures increase in tem can cause pseudonormalization of the E/A ratio (>1). The normal appearing E wave reflects the increase in passive filling from the increased left atrial-left ventricular gradient. The normal appearing A wave reflects the decreased contribution of the atrial contraction.
- Deceleration time: Describes the time it takes for the E wave velocity to become zero (normal is 220 milliseconds). In impaired relaxation, it exceeds 240 ms.