A variety of “add-on devices” are used with infusion therapy. They are defined as additional components added to the administration set or VAD. Add-on devices include the following:
In general, add-on devices are used only when clinically indicated for a specific purpose and should be of a design that ensures a secure junction, reduces manipulation, and reduces the risk of disconnection (Gorski et al., 2021, p. 107).
Extension Sets
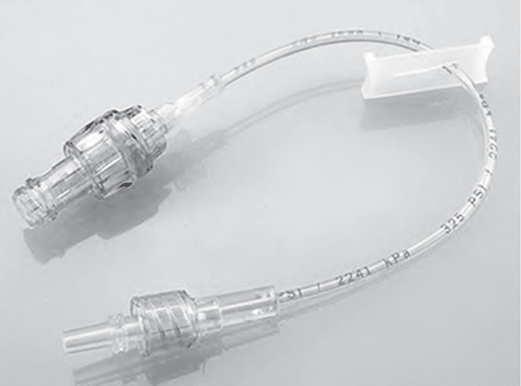
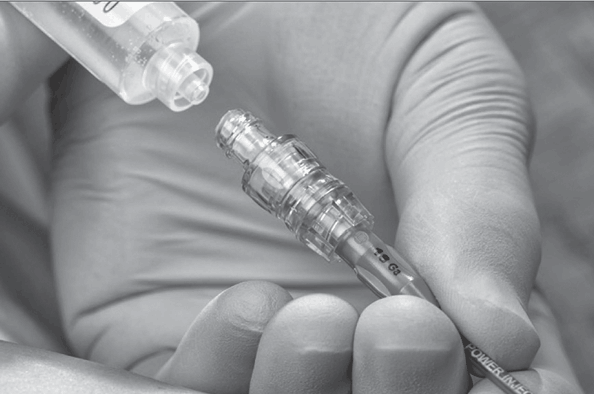
Extension tubing may be added to the administration set or VAD. Extension sets may be straight or in a Y configuration or may have multiple entries. Straight extension sets may be connected to the VAD with an NC added to the end of the set to increase the length (Fig. 5-15). This is common with home-care patients, for whom the extra length allows easier access to disinfect the NC and self-infuse a medication. Y-configured extension sets usually have a clamp on both segments and can be added to allow simultaneous or separate administration of solutions. Multientry extension sets may have three or more “pigtails” allowing entry into the infusion system. When used with power-injectable catheters, an extension set with such capability must be used (Fig. 5-16).
A catheter connection device such as a J loop or T connector added to a PIVC makes it easier to convert from a continuous to an intermittent infusion with less manipulation at the catheter insertion site.
Stopcocks
A stopcock is a device that controls the direction of flow of an infusate through manual manipulation of a direction-regulating valve. A stopcock is usually a three- or four-way device. A three-way stopcock connects two lines of fluid to a patient and provides a mechanism for either one to run to the patient (similar to a faucet) (Fig. 5-17). With a four-way stopcock, the valve can be manipulated so that one or both lines can run to the patient, either alone or in combination.
When the stopcock ports are uncapped, they are vulnerable to touch contamination. The stopcock itself is small and requires handling in such a way that sterility can easily be compromised. The INS Standards recommend the use of a stopcock with an integrated NC rather than a solid cap or replace the solid caps with NCs (Gorski et al., 2021, p. S107). Adherence to ANTT is essential with each access and change of add-on devices.
INS Standard Change the add-on device with new VAD insertion, with each administration set replacement if integrated tubing design, or as defined by the organization, and whenever the integrity of the product is compromised or suspected of being compromised (Gorski et al., 2021, p. S107).
 NURSING FAST FACT!
NURSING FAST FACT!Caution should be exercised when using a stopcock because of the risk of contamination of the IV system and therefore increasing the risk for a bloodstream infection. |
Needleless Connectors
An NC is attached to the hub of the PIVC or CVAD, allowing the tip of a syringe or male Luer end of the IV administration set to be attached. Although needleless connector is the current term used by INS and others, other commonly used terms are needlefree connectors, injection caps, injection ports, and valves. NCs allow for venous access without removal of the connector, thus maintaining a closed infusion system.
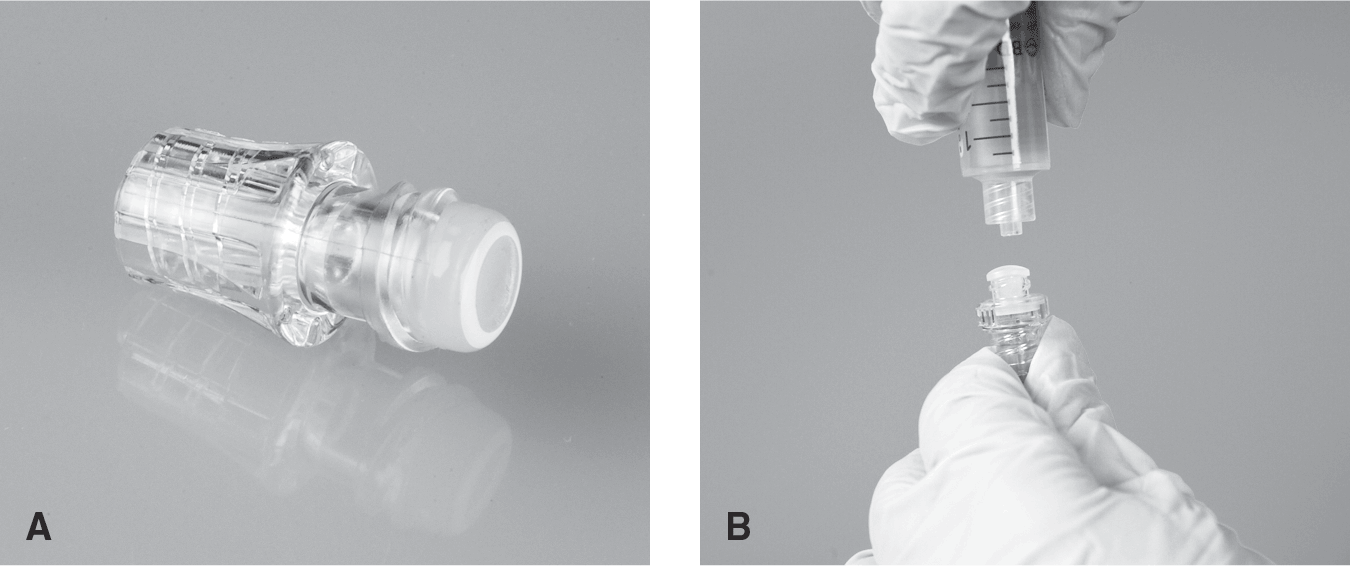
Many types of NCs are available, and it is important to understand how they work and their impact on both potential infection and VAD occlusion. NCs work via different mechanisms such as a split septum accessed with a blunt cannula (Fig. 5-18AB) or a mechanical or pressure-sensitive valve (Fig. 5-19). It is important that the nurse understand what type of NC is used in the organization and to follow the proper clamping sequence, in accordance with manufacturer's directions for use, to reduce blood reflux into the catheter tip (Table 5-1). Reflux of blood within the catheter lumen may lead to catheter occlusion, although the time to coagulate/volume of blood unknown (Gorski et al., 2021). It is important to recognize that with smaller catheter lumens and a greater surface area, such as with PICCs and midline catheters, there will be a greater distance of reflux into lumen, potentially increasing the risk for occlusion.
| Type of NC | Description | Clamping Sequence |
|---|---|---|
| Negative displacement | Blood refluxes into the catheter lumen when IV tubing or a syringe is disconnected before the VAD is clamped or when an infusion container runs dry. A positive fluid displacement technique is required to overcome blood reflux. | Flush VAD, clamp the VAD, then disconnect the flush syringe |
| Positive displacement | With these devices, an internal reservoir within the NC holds a small amount of fluid. When the syringe or IV tubing is disconnected, fluid is pushed out to overcome blood reflux. | Flush VAD, disconnect the flush syringe, then clamp |
| Neutral displacement | An internal mechanism is designed to reduce blood reflux upon connection or disconnection. However, studies have demonstrated that there is reflux with neutral NCs and it is suggested that neutral is not an accurate label (Gibson & Primeaux, 2020; Hull et al., 2018) | Flush VAD, clamp the VAD, then disconnect the flush syringe |
| Antireflux | A three-position pressure-activated valve opens and closes based on infusion pressure. | No specific clamping sequence |
Because NCs are a known source of contamination, attention to disinfection when accessing the NC for VAD flushing and medication administration is critical in reducing the intraluminal introduction of microbes. Guidance and options for disinfection are addressed in Chapter 2.
Changing the NC
The NC is changed no more frequently than every 72 to 96 hours, as changing more often adds no benefit and has been shown to increase the risk of central line-associated bloodstream infection (Gorski et al., 2021, p. S106). Additionally, the NC should be changed in the following circumstances:
- When it is removed for any reason
- If residual blood or debris is present
- Prior to drawing a blood sample for culture
- Upon contamination
- When the administration set is changed, if the NC is used with a continuous infusion
- According to organizational policies and procedures
- According to manufacturer's directions for use (Gorski et al., 2021, p. S106)
There is a lack of studies addressing home care patients. It is typical practice to routinely change the NC every 7 days with intermittent infusions or more often based on INS criteria as listed above (Gorski, 2017).
 NURSING FAST FACT!
NURSING FAST FACT!Blood reflux, and thus the risk for catheter occlusion, is dependent on proper flushing and clamping technique based on whether the connector is classified as a positive, negative, neutral, or antireflux NC. It is important that all nurses understand which type of device is being used. |
 NURSING FAST FACT!
NURSING FAST FACT!For all catheters that are supplied with a clamp, the clamp should be closed when it is not in use to prevent the risk of air embolism or exsanguination with accidental dislodgement of the needleless connector. |
Filtration
Filters may be used during the infusion of IV solutions to prevent the administration of any particulate matter, air, microorganisms, or endotoxins that may be in the infusion system. Unwanted matter is present in all types of solutions, and the United States Pharmacopeia (USP) has set standards regarding the amount of particulate matter allowable. The addition of medications and administration sets potentially increases the amount of particulate matter. Organ damage from particles includes capillary blockage; activation of platelets, neutrophils, and endothelial cells that can result in microthrombi; and deterioration of the microcirculation (Boehne et al., 2013). Filtration in critically ill and neonatal patients is an important area of continued research.
In a review of the literature related to air in infusion systems, the authors concluded that although air embolism is a relatively rare event, patients may be at risk from even small air bubbles (Wilkins & Unverdorben, 2012). The INS Standards recommend considering the use of filtration to reduce microbubbles which are particularly common in patients undergoing procedures such as hemodialysis and cardiopulmonary bypass (Forsberg et al., 2019; Gorski et al., 2021).
Filtration is always used with blood transfusions, PN solutions, ILE, intraspinal infusions, and specific medications/solutions as recommended by the manufacturer in the medication instructions for use.
Filters are available as add-on devices or as inline components as an integral part of the administration set. Advantages to inline filters include reduced risk for contamination and no risk of filter-tubing separation. Disadvantages include the need for an entire administration set change should the filter clog. The location of the filter may also be a disadvantage. If the filter is located at the upper portion of the tubing, it retains only substances that enter the tubing above the filter. Add-on filters can be changed if they become clogged and should be placed at the distal end of the tubing. As with all infusion procedures, attention to ANTT is critical when manipulating the infusion system.
Inline filters are available in a variety of forms, sizes, and materials. Common filter sizes used with common IV solutions and medications are:
- 0.2 micron: Most common, air eliminating, bacterial retentive
- 0.45 micron: Remove fungi or bacteria, may be air eliminating
- 1.2 micron: Used with PN solutions and lipid emulsions (see Chapter 12)
Examples of filters are shown in Figures 5-20, 5-21, and 5-22.

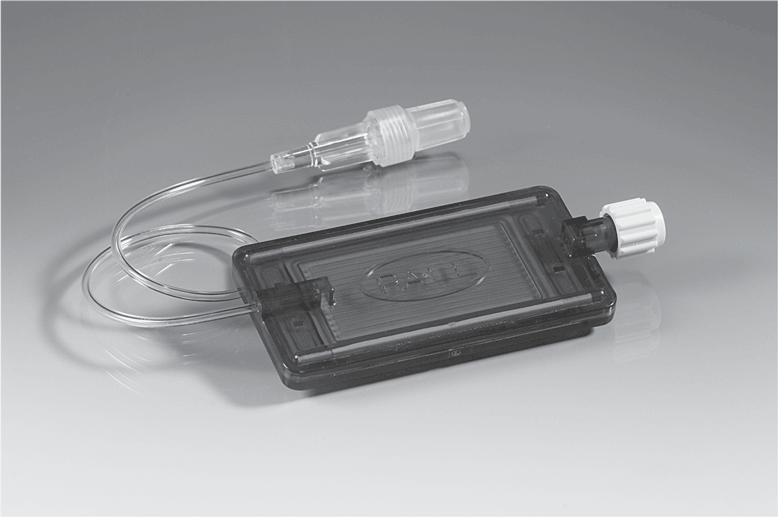
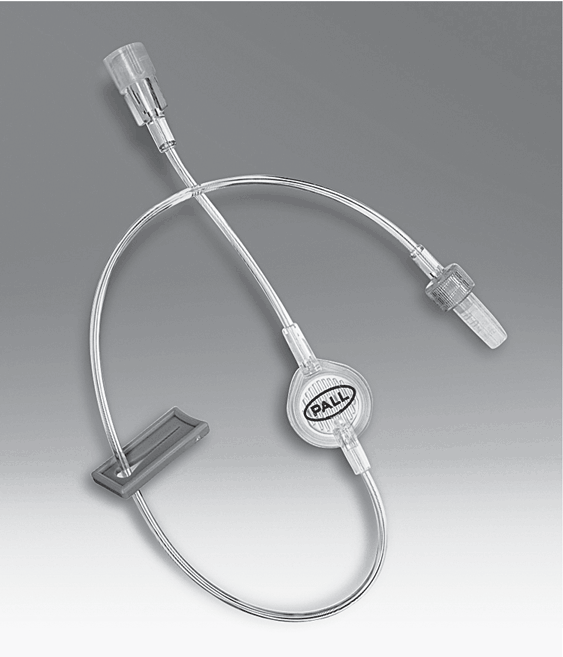
INS Standard Locate the inline filter on the administration set as close to the VAD hub as possible; add-on components (e.g., extension set) below or after the filter will result in additional particulate matter infusing to the patient (Gorski et al., 2021, p. S102).
Filters range in size from 170-260 microns (largest blood filters) to 0.2 micron (smallest). They allow liquids but not particles to pass through. The finer the membrane, the more fully it will filter the liquid. A 5-micron screen will retain on the flat portion of the membrane all particles larger than 5 microns. A 0.2-micron filter is used to retain bacteria, fungi, and air.
A 0.2-micron filter is contraindicated with administration of blood or blood components and lipid emulsions. Other contraindications include the administration of low-volume medications (total amount <5 mL over 24 hours), IV push medications, medications with pharmacological properties that are altered by the filter membrane, and medications that adhere to the filter membrane.
All filters have a certain pressure value at which they will allow the passage of air from one side of a wetted hydrophilic membrane to the other. Filters are also rated according to the pounds per square inch (psi) of pressure they can withstand. The filter should withstand the psi exerted by the infusion pump, or rupture may occur. If the psi rating of the housing is less than that of the filter membrane, excess force will break the housing.
Blood Filters
In accordance with the American Association of Blood Banks, blood components must be transfused through special tubing with a filter designed to remove blood clots and potentially harmful particles (AABB, 2018). Blood administration sets have a standard clot filter of 170 to 260 microns. These filters are intended to remove coagulated products, microclots, and debris resulting from collection and storage. Commercially available filters include the standard clot filter, the microaggregate filter, and the leukocyte reduction filter. Refer to Chapter 11 for information related to filtration in transfusion therapy.