Symptoms
Pain, decreased vision, inability to open the eyelids due to severe swelling, recent history of trauma or surgery to the eyelids or orbit, and history of anticoagulation. Because the orbit is a bony compartment with firm anterior soft tissue tethering by the orbital septum, any process (blood, pus, air) that rapidly fills the orbit results in a compartment syndrome. As pressure builds within the orbit, ischemia to the optic nerve, globe, and retina occur, potentially resulting in devastating, permanent visual loss. OCS is an ophthalmic emergency.
Most iatrogenic postoperative orbital hemorrhages evolve within the first 6 hours following surgery. |
Signs
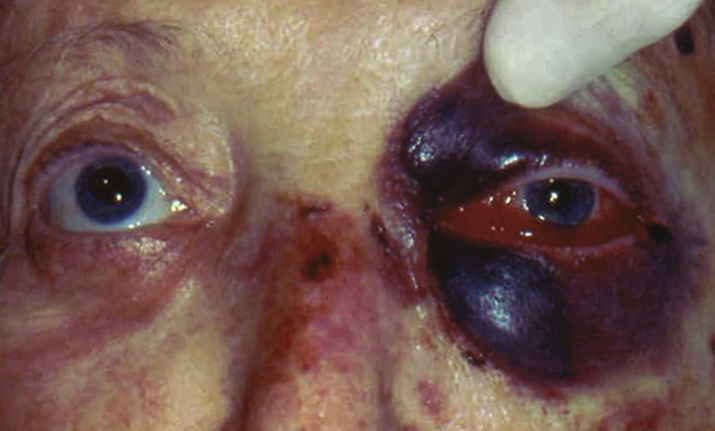
(See Figure 3.10.1.)
Critical
Proptosis with resistance to retropulsion, tense (“rock hard”) eyelids that are difficult to open, vision loss, afferent pupillary defect, dyschromatopsia, and increased IOP.
Other
Eyelid ecchymosis, diffuse subconjunctival hemorrhage, chemosis, congested conjunctival vessels, evidence of disc swelling from compressive optic neuropathy or retinal vascular occlusion, and limited extraocular motility in any or all fields of gaze.
Differential Diagnosis
- Orbital cellulitis: Fever, proptosis, chemosis, limitation, or pain with eye movement; also may follow trauma, but usually not as acutely. A rapidly expanding orbital abscess may result in OCS, and in such cases, the acute management is the same as that for orbital hemorrhage. See 7.3.1, ORBITAL CELLULITIS.
- Severe orbital emphysema (“tension pneumo-orbit”): Tight orbit, tight eyelids, crepitus, and decreased extraocular motility; may follow orbital fracture with or without nose blowing. See 3.9, ORBITAL BLOWOUT FRACTURE.
- Orbital fracture: Limited extraocular motility, enophthalmos, or proptosis may be present. See 3.9, ORBITAL BLOWOUT FRACTURE.
- Ruptured globe: Subconjunctival edema and hemorrhage may mask a ruptured globe. See 3.14, RUPTURED GLOBE AND PENETRATING OCULAR INJURY.
- High-flow (direct) carotid–cavernous fistula: May follow trauma either acutely or subacutely; most are spontaneous and nontraumatic. Pulsatile exophthalmos, ocular/brow bruit, corkscrew arterialized conjunctival vessels, chemosis, and increased IOP may be seen. Usually unilateral, although bilateral signs from a unilateral fistula may be seen occasionally. See 10.10, CAVERNOUS SINUS AND ASSOCIATED SYNDROMES (MULTIPLE OCULAR MOTOR NERVE PALSIES).
- Varix: Increased proptosis with Valsalva maneuver. Not usually seen acutely after trauma, and in the vast majority of orbital varices, there is no history of trauma or surgery.
- Lymphangioma: Usually in younger patients. May have acute proptosis, ecchymosis, and external ophthalmoplegia after minimal trauma or upper respiratory tract infection. MRI is usually diagnostic.
- Spontaneous retrobulbar hemorrhage: Signs and symptoms are identical to those of traumatic or postoperative hemorrhage. May occur in patients who are chronically anticoagulated or with an underlying coagulopathy from other systemic disease (e.g., hemophilia). Occasionally reported in pregnant women, especially during labor. Typically seen as a subperiosteal hematoma along the orbital roof and may be misdiagnosed as an orbital neoplasm. MRI is helpful in identifying blood-breakdown products (see 14.3, MAGNETIC RESONANCE IMAGING).
Workup
- Complete ophthalmic examination; check specifically for an afferent pupillary defect, loss of color vision, the degree of tightness of the eyelids, resistance to retropulsion, increased IOP, pulsations of the central retinal artery (often precedes artery occlusion), choroidal folds (sign of globe distortion from severe optic nerve stretching), and optic nerve edema. Note that visual function may span from normal to no light perception.
- CT scan of the orbit (axial, coronal, and parasagittal views). When vision and/or optic nerve function are threatened, CT should always be delayed until definitive treatment with canthotomy/cantholysis. CT rarely shows a discreet hematoma. Typically, retrobulbar hemorrhage manifests as a diffuse, increased reticular pattern of the intraconal orbital fat. The so-called teardrop or tenting sign may be seen: the optic nerve is at maximum stretch and distorts (tents) the back of the globe into a teardrop shape. This is an ominous radiologic sign, especially if the posterior scleral angle is <130 degrees. The presence of an orbital fracture may help to decompress the orbit and decrease, but not rule out, the possibility of OCS. It is important to remember that patients can still develop OCS and optic neuropathy even with large orbital fractures, as blood may simply fill the adjacent paranasal sinus and then cause an OCS.
Retrobulbar hemorrhage with OCS is a clinical diagnosis and does not require imaging. A delay while CT is being obtained may cause further visual compromise. Imaging can be obtained once the OCS has been decompressed and visual function stabilized. |
Treatment
The key to effective management of clinically significant retrobulbar hemorrhage with OCS is timely and aggressive soft tissue decompression. The initial goal is to decrease the compression on critical soft tissues, mainly the optic nerve. All patients should be treated utilizing the same guidelines, even if the hemorrhage is thought to have occurred hours or days ago, since it is often impossible to know at what point along the clinical timeline optic neuropathy manifested.
- If optic neuropathy is present, immediately release orbital pressure with lateral canthotomy and inferior cantholysis (see Figure 3.10.2 below). The procedure can be performed in an office or ER setting with basic instrumentation and local anesthetic injection if possible. Conscious sedation can be used in the ER setting if this does not delay treatment, but is usually unnecessary.
- If there are no orbital signs and no evidence of ocular ischemia or compressive optic neuropathy but the IOP is increased (e.g., ≥35 mm Hg in a patient with a normal optic nerve, or ≥20 mm Hg in a patient with glaucoma who normally has a lower IOP), the patient is treated in a more stepwise fashion in an effort to reduce the IOP (see below and 9.1, PRIMARY OPEN ANGLE GLAUCOMA).
3-10.2 Lateral canthotomy and cantholysis.
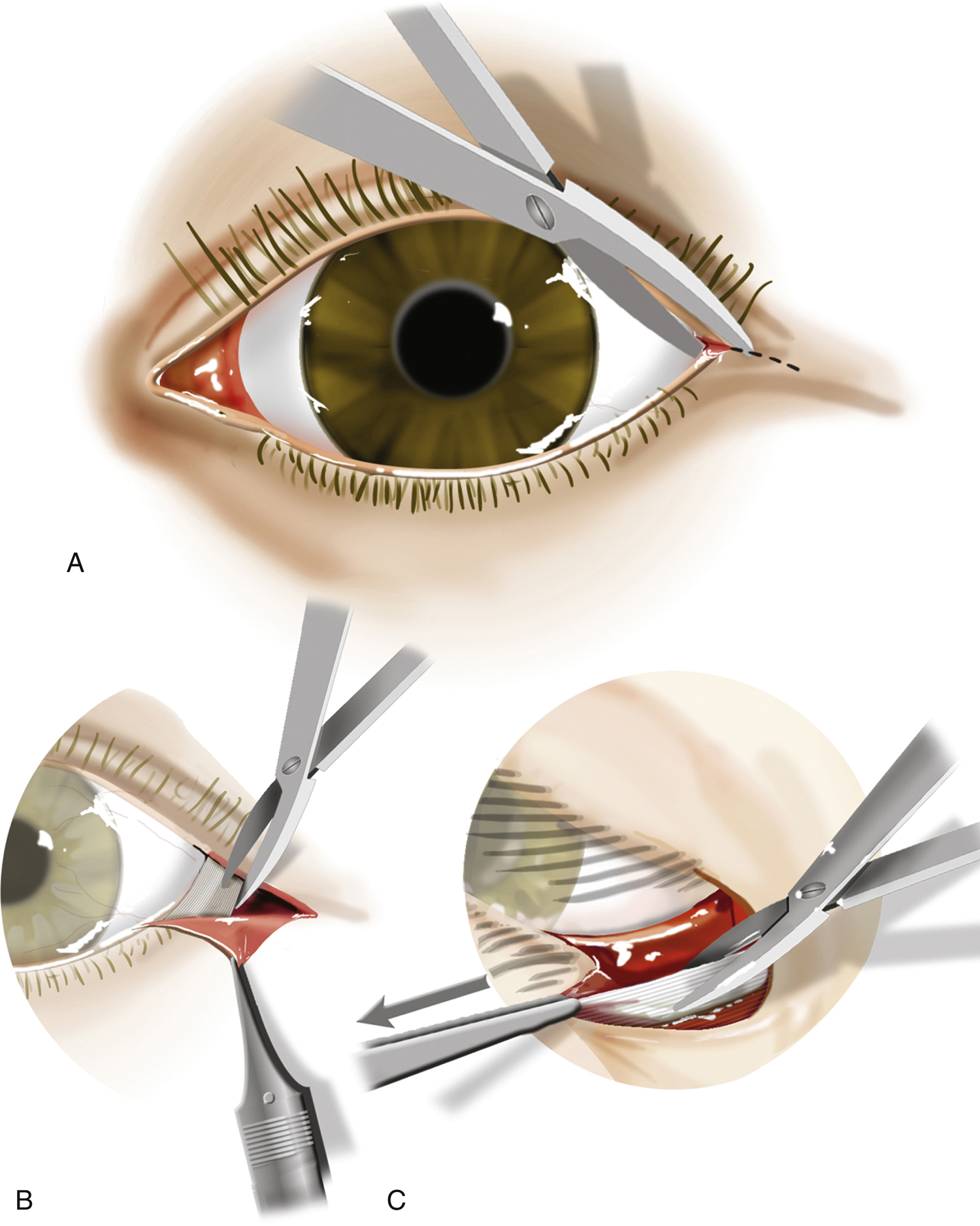
A:Lateral canthotomy.B:Grasp the lateral lower eyelid with toothed forceps.C:Pull the eyelid anteriorly. Point the scissors toward the patient’s nose, strum the lateral canthal tendon, and cut.
Canthotomy and Cantholysis
(See Figure 3.10.2.)
The goal of canthotomy and cantholysis is to perform an adequate soft tissue decompression of the orbit by disinserting the lower eyelid from its periosteal attachments. A nonperfused retina infarcts irreversibly in approximately 90 minutes, and a delay of definitive treatment of longer duration may lead to retinal nerve fiber death and permanent vision loss.
A canthotomy alone is inadequate treatment. A cantholysis must be performed. |
- Consider subdermal injection of lidocaine 2% with epinephrine (inject away from the eye). Because of the eyelid edema and acidotic local environment, local anesthesia may not be effective. A field block may be used. The patient should be warned that the procedure may be painful, but fortunately in most cases, canthotomy and cantholysis can be performed quickly.
- Consider placing a hemostat horizontally at the lateral canthus and clamp for 1 minute to compress the tissue and reduce bleeding (an optional step that should not be performed without good local anesthesia).
- Only two instruments are needed for canthotomy and cantholysis: A pair of blunt-tipped scissors (e.g., Westcott or Stevens) and forceps with heavy teeth (e.g., Bishop Harmon or Adson). Avoid sharp-tipped scissors to minimize the chance of globe injury. Delicate forceps (e.g., 0.12-mm Castroviejo) will not provide enough traction to effectively disinsert the eyelid and should not be used.
- Perform the canthotomy. Place the scissors across the lateral canthus and incise the canthus for about 1-cm full thickness (from conjunctiva to skin). Forceps are not needed for this step. This step simply gains access to the inferior crux of the lateral canthal tendon. It provides little soft tissue decompression.
- Perform inferior cantholysis. With the toothed forceps, grasp the lower eyelid at the inner edge of the incised canthus. With the patient supine, traction should be directed upward, toward the ceiling. Place the scissors in an open position just beneath the skin, with the tips pointing toward the tip of the nose, and begin to cut. Some advocate “strumming” of the lateral canthal tendon, but this is not essential and sometimes difficult to assess because of tissue edema. As the canthal tendon is released, the eyelid should come completely away from the globe.
- There are several clues to a successful cantholysis. The eyelid should release completely away from the globe. Once the forceps are released, the lateral portion of the eyelid margin should move medially, usually to the lateral aspect of the limbus of the globe. If any eyelid margin still remains in its normal position lateral to the temporal limbus, the cantholysis is incomplete: keep cutting!
- The incision will bleed; however, cautery is usually unnecessary. Pressure over the bone of the lateral orbital rim (but not on the globe and orbit) for several minutes usually results in good control. However, with the widespread use of anticoagulant and antiplatelet medications (e.g., warfarin, aspirin, clopidogrel) as well as increasing prescription of irreversible anticoagulants (e.g., rivaroxaban, dabigatran, etc.), excessive bleeding can be encountered. In these cases, manual pressure is often inadequate and applying hemostatic aids (e.g., biologic agents such as thrombin and fibrinogen or physical agents such as gelatin, collagen, cellulose) may be necessary. Cautery is effective if available.
- The results of a successful cantholysis are usually evident within the first 15 minutes. IOP should decrease, and the retina should reperfuse. Cadaver studies have shown a reliable correlation between IOP and intraorbital pressure. A significant decrease in IOP after cantholysis is indicative of a successful orbital soft tissue decompression. Depending on the timing of the cantholysis in relation to the hemorrhage, vision may dramatically improve. Superior cantholysis is usually unnecessary: cadaver studies have not shown a dramatic decrease in intraorbital pressure with superior cantholysis. In addition, superior cantholysis may result in lacrimal gland incision, which can bleed profusely. By far the most common reason for persistent signs of OCS following inferior cantholysis is inadequate cantholysis. Make sure the cantholysis is performed effectively.
- 3.Close observation is indicated in all cases of retrobulbar hemorrhage, including those that have not yet affected visual function. It is therefore dangerous to assume that a patient with recent injury/surgery, retrobulbar hemorrhage, and normal optic nerve function is stable enough for discharge. In such cases, it is best to follow the patient for 4 to 6 hours with serial examinations in the ER or hospital. If a patient presents with no evidence of OCS 6 or more hours after the initial insult, it is reasonable to discharge the patient with specific instructions (see follow up). For patients presenting with OCS, see NOTE below.
- 4.Anticoagulants (e.g., warfarin) and antiplatelet agents (e.g., aspirin) are often discontinued, if possible, to prevent rebleeding. This decision must be made in conjunction with an internist or cardiologist. Intravenous corticosteroids may be indicated to further decrease soft tissue edema when no TBI is present. Antibiotics may be indicated, depending on the etiology of the hemorrhage. Frequent ice compresses (20 minutes on, 20 minutes off) are important, and their compliant use should be emphasized to the patient and the nursing staff.
- 5.Medical IOP control as needed (see 9.1, PRIMARY OPEN ANGLE GLAUCOMA and 9.4, ACUTE ANGLE CLOSURE GLAUCOMA for treatment indications/contraindications):
- Topical IOP-lowering agents such as beta-blockers, alpha-agonists, or carbonic anhydrase inhibitors.
- Oral carbonic anhydrase inhibitor (e.g., acetazolamide).
- Hyperosmotic agent (e.g., mannitol).
The cantholysis is critical to decompress the orbit and is done exclusively by feel. Do not visually search for a specific tendon or anatomic landmark. |
The efficacy of inferior cantholysis for OCS is based on the assumption that at the time of the procedure, all active orbital bleeding has tamponaded; this is indeed the case in the majority of retrobulbar hemorrhages. However, if active bleeding persists, the OCS will recur as the blood fills the decompressed orbit. For this reason, it is prudent to monitor patients who present with OCS and optic neuropathy for 12 to 24 hours following cantholysis. |
When an OCS exists in a situation where canthotomy/cantholysis cannot immediately be completed, the use of IV mannitol may serve as a temporizing measure and assist in lowering intraocular and intraorbital pressure based on a recent study in which primates were subjected to experimental orbital hemorrhage. However, there should be no systemic or intracranial contraindications to mannitol before administration. Note that IV mannitol is not a substitute for definitive management of OCS by canthotomy/cantholysis! |
Follow Up
In cases where vision is threatened, monitor the patient closely until stable, with frequent vision and IOP checks.
Any patient with a posttraumatic orbital hemorrhage older than 6 to 8 hours with normal visual function should be instructed in detail on how to serially measure visual function, especially over the first 24 hours, and to return immediately if vision deteriorates. Cantholysis wounds may be left open with application of antibiotic ointment t.i.d. to spontaneously heal, or closed with a secondary canthoplasty 1 to 3 weeks later. If reconstruction of the lateral canthus is indicated, it may be performed as an outpatient procedure under local anesthesia. The inferior canthal tendon is sutured back to the inner aspect of the lateral orbital rim. Interestingly, a significant percentage of eyelids will heal adequately without any surgery. If a residual optic neuropathy is present, the patient should be followed with serial examinations and visual fields. It is not uncommon for visual function to continue to improve over the first 6 months.