Diabetic Retinopathy Disease Severity Scale
No apparent retinopathy.
Mild nonproliferative diabetic retinopathy (NPDR): Microaneurysms only.
Moderate NPDR: More than mild NPDR, but less than severe NPDR (See Figure 11.12.1). May have CWSs and venous beading.
Figure 11.12.1: Moderate nonproliferative diabetic retinopathy with microaneurysms and cotton–wool spots.
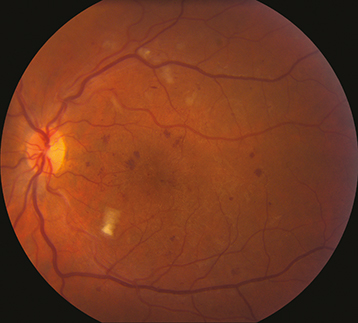
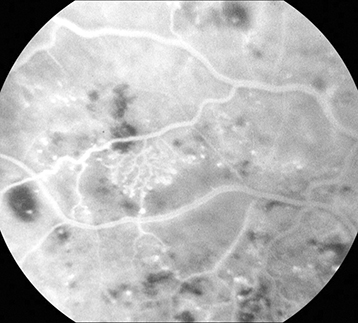
Severe NPDR: Any of the following in the absence of PDR: Diffuse (traditionally >20) intraretinal hemorrhages in all 4 quadrants, ≥2 quadrants of venous beading, or ≥1 quadrant of prominent intraretinal microvascular abnormalities (See Figure 11.12.2).
Figure 11.12.2: Intravenous fluorescein angiography of intraretinal microvascular abnormality.
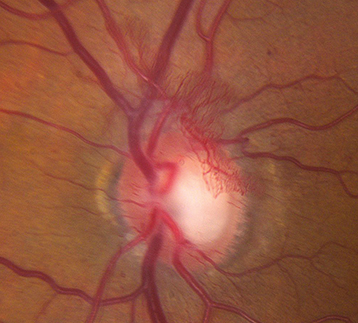
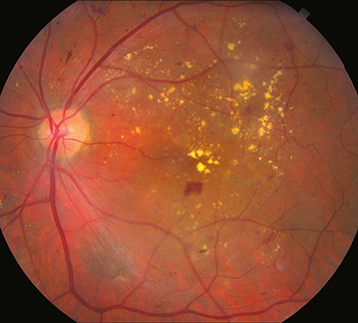
PDR: Neovascularization of any of the following: iris, angle, optic disc, or elsewhere in retina; or vitreous/preretinal hemorrhage (See Figures 11.12.3 and 11.12.4).
Figure 11.12.3: Proliferative diabetic retinopathy with neovascularization and scattered microaneurysms.
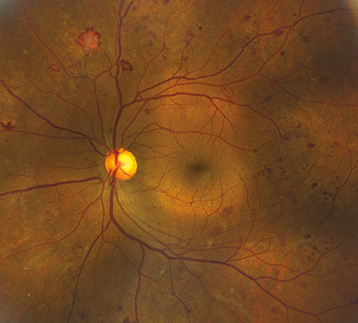
Figure 11.12.4: Proliferative diabetic retinopathy with neovascularization of the optic disc.
Diabetic macular edema (DME): May be present in any of the stages. DME affecting or threatening the fovea is an indication for treatment (See Figure 11.12.5).
Figure 11.12.5: Nonproliferative diabetic retinopathy with clinically significant macular edema.
Differential Diagnosis for Nonproliferative Diabetic Retinopathy
CRVO: Optic disc swelling, veins are more dilated and tortuous, hard exudates and microaneurysms usually not found, hemorrhages are nearly always in the NFL (“splinter hemorrhages”). CRVO is generally unilateral and of more sudden onset. See 11.8, Central Retinal Vein Occlusion.
BRVO: Hemorrhages are distributed along a vein and generally respect the horizontal raphe. See 11.9, Branch Retinal Vein Occlusion.
OIS: Hemorrhages mostly in the midperiphery and larger; exudates are absent. Usually accompanied by pain; mild anterior chamber reaction; corneal edema; episcleral vascular congestion; a mid-dilated, poorly reactive pupil; iris neovascularization. See 11.11, Ocular Ischemic Syndrome/Carotid Occlusive Disease.
Hypertensive retinopathy: Hemorrhages fewer and typically flame shaped, microaneurysms rare, and arteriolar narrowing present often with arteriovenous crossing changes (“AV nicking”). See 11.10, Hypertensive Retinopathy.
Radiation retinopathy: Usually develops within a few years of radiation. Microaneurysms are rarely present. See 11.5, Cotton–Wool Spot.
Differential Diagnosis for Proliferative Diabetic Retinopathy
Neovascular complications of CRAO, CRVO, or BRVO: See specific sections.
Sickle cell retinopathy: Peripheral retinal neovascularization with “sea fan” configuration. See 11.20, Sickle Cell Retinopathy (Including Sickle Cell Disease, Anemia, and Trait).
Embolization from intravenous drug abuse (talc retinopathy): Peripheral retinal neovascularization in patient with history of intravenous drug abuse. Typically see talc particles in retinal vessels. See 11.33, Crystalline Retinopathy.
Sarcoidosis: May have uveitis, exudates around veins (“candle-wax drippings”), NVE, or systemic findings. See 12.5, Retinal Vasculitis.
Other inflammatory syndromes (e.g., systemic lupus erythematosus).
OIS: See 11.11, Ocular Ischemic Syndrome/Carotid Occlusive Disease.
Radiation retinopathy: See above.
Hypercoagulable states (e.g., antiphospholipid syndrome).
Systemic health status should be optimized: strict control of diabetes with lifestyle modifications or pharmacologic agents; goal HbA1c <7%.
Diabetic Macular Edema
Anti-VEGF agents (FDA-approved ranibizumab 0.5 mg, brolucizumab 6 mg, faricimab 6 mg, aflibercept 2 mg, and high-dose aflibercept 8 mg, as well as off-label bevacizumab) are first-line therapy for center-involving DME.
Those patients who have a suboptimal response to these anti-VEGF agents or require ongoing, frequent anti-VEGF therapy can consider intravitreal corticosteroid therapy with FDA-approved dexamethasone or long-acting fluocinolone acetonide injectable implants. Off-label intravitreal corticosteroid (e.g., triamcinolone 40 mg/mL, injecting 1 to 4 mg) can also be considered. Complications include cataract formation and elevated IOP.
Focal macular laser treatment can be considered in patients with extrafoveal microaneurysms causing significant edema. Macular laser can also be considered in patients for whom there is a suboptimal response to pharmacotherapy. Most practitioners avoid using anti-VEGF agents in pregnant patients, though no study has definitively shown adverse fetal side effects.
Proliferative Diabetic Retinopathy
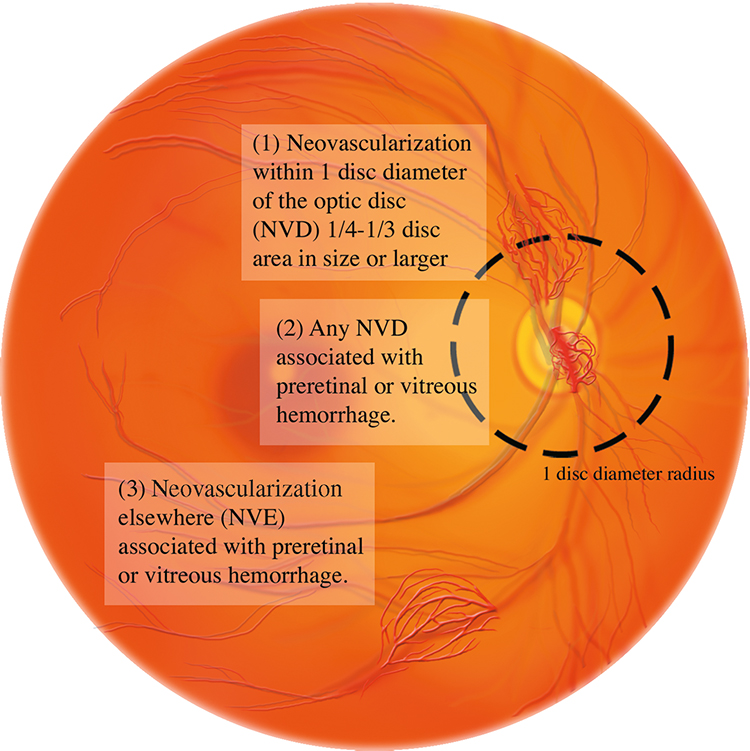
PRP is indicated for any one of the following high-risk characteristics (See Figure 11.12.6):
NVD greater than one-fourth to one-third of the disc area in size.
Any degree of NVD when associated with preretinal hemorrhage or VH.
NVE greater than one-half of the disc area in size when associated with preretinal hemorrhage or VH.
Any NVI or NVA.
Figure 11.12.6: High-risk characteristics for diabetic retinopathy.
Anti-VEGF therapy can be utilized for PDR as an alternative to PRP and is the preferred initial therapy in the presence of DME or if the view to the peripheral retina is limited by VH. Anti-VEGF therapy without PRP should be utilized judiciously, as patients’ nonadherence to recommended treatment intervals have worse anatomic and visual outcomes.
|
 NOTE NOTESome physicians treat NVE or any degree of NVD without preretinal hemorrhage or VH, especially in patients that may not have consistent follow-up. |
Indications for Vitrectomy
Vitrectomy may be indicated for any one of the following conditions:
Dense, nonclearing, or recurrent VH or premacular hemorrhage causing significant decreased vision.
Traction RD involving the macula.
Macular epiretinal membranes (ERMs) or vitreomacular traction (VMT) causing significant visual symptoms.
Chronic DME not responsive to other treatment.
|
 NOTE NOTEB-scan US may be required to rule out tractional detachment of the macula in eyes with dense VH obscuring a fundus view. |





